2. Description of the Survey
2.1 Sample Design
The respondent universe for the National Survey on Drug Use and Health (NSDUH) is the civilian, noninstitutionalized population aged 12 years or older residing within the United States. The survey covers residents of households (e.g., individuals living in houses or townhouses, apartments, and condominiums; civilians living in housing on military bases) and individuals in noninstitutional group quarters (e.g., shelters, rooming or boarding houses, college dormitories, migratory workers’ camps, halfway houses). Excluded from the survey are individuals with no fixed household address (e.g., homeless and/or transient people not in shelters), active-duty military personnel, and residents of institutional group quarters, such as correctional facilities, nursing homes, mental institutions, and long-term care hospitals.
2.1.1 Coordinated Sample Design for 2014 through 2022
A coordinated sample design was developed for the 2014 through 2022 NSDUHs. The coordinated sample design is state-based, with an independent, multistage area probability sample within each state and the District of Columbia. States were the first level of stratification. As shown in Figure 2.1, each state was further stratified into approximately equally populated state sampling regions (SSRs). Creation of the multistage area probability sample then involved selecting census tracts within each SSR (Stage 1), census block groups within census tracts (Stage 2), and area segments (i.e., a collection of census blocks) within census block groups (Stage 3). Finally, dwelling units (DUs) were selected within segments (Stage 4), and (within each selected DU) up to two residents who were at least 12 years old were selected for the interview (Stage 5).
The coordinated sample design for 2014 through 2022 includes a 50 percent overlap in third-stage units (area segments) within each successive 2-year period from 2014 through 2022. DUs not sampled the first year are eligible for selection the following year. There is no planned overlap of sampled residents. However, individuals may be selected in consecutive years if they move and their new residence is selected the year after their original DU was sampled. The planned overlap in area segments reduces annual costs. When trend data are reported, this sample overlap also slightly increases the precision of estimates for year-to-year trends because of the expected small but positive correlation resulting from the overlapping area segments between successive survey years.
The 2014 through 2022 NSDUH sample design provides sufficient sample sizes to support state and national estimates. The cost-efficient sample design allocates completed interviews (and associated sample) to the largest 12 states approximately proportional to the size of the civilian, noninstitutionalized population aged 12 or older in these states. In the remaining states, a minimum sample size is required to support reliable state estimates by using either direct methods (by pooling multiple years of data) or small area estimation.2 Population projections based on the 2010 census and data from the 2006 to 2010 American Community Surveys (ACSs) were used to construct the sampling frame for the 2014 through 2022 NSDUHs.
Table 2.1 at the end of this chapter shows the targeted numbers of completed interviews in selected states per year for the 2014 through 2022 samples.3 For Hawaii, the sample was designed to yield a minimum of 200 completed interviews in Kauai County, Hawaii, over a 3-year period. To achieve this goal while maintaining precision at the state level, the annual sample in Hawaii consists of 67 completed interviews in Kauai County and 900 completed interviews in the remainder of the state, for a total of 967 completed interviews each year for 2014 onward. The sample design also targeted 960 completed interviews in each of the remaining 37 states and the District of Columbia that are not listed individually in Table 2.1.
2.1.1.1 Selection of Area Samples and Dwelling Units within States
As mentioned previously, states were first stratified into SSRs. The number of SSRs varied by state and was related to the state’s sample size. SSRs were contiguous geographic areas designed to yield approximately the same number of interviews within a given state.4 A total of 750 SSRs are in the 2014 through 2022 sample design. Table 2.1 also shows the number of SSRs for different states.
The first stage of selection for the 2014 through 2022 NSDUHs was census tracts.5 Within each SSR, 48 census tracts6 were selected with probability proportional to a composite measure of size.7 This stage was included to contain sampled areas within a single census tract to the extent possible in order to facilitate merging to external data sources. Within sampled census tracts, adjacent census block groups were combined as necessary to meet the minimum DU size requirements.8 One census block group or second-stage sampling unit then was selected within each sampled census tract with probability proportional to population size. The selection of census block groups at the second stage of selection is included to facilitate possible transitioning to an address-based sampling design in a future survey year. For the third stage of selection, adjacent blocks were combined within each sampled census block group to form area segments. One area segment was selected within each sampled census block group with probability proportionate to a composite measure of size.
Although only 40 segments per SSR were needed to support the coordinated 9-year sample for the 2014 through 2022 NSDUHs, an additional 8 segments per SSR were selected to support a number of large field tests.9 Eight sample segments per SSR were fielded during the 2020 survey year. Four of these segments were selected for the 2019 survey and were used again in the 2020 survey; four were selected for the 2020 survey and will be used again in the 2021 survey.
Sampled segments for 2020 were allocated equally into four separate samples, one for each 3-month period (calendar quarter) during the year. That is, a sample of addresses was selected from two segments in each calendar quarter.10 In each of the area segments, a listing of all addresses was made, from which a national sample of 642,549 addresses was selected. Of the selected addresses, 536,203 were determined during the field period to be eligible sample units. In these sample units (which can be either households or units within group quarters), sampled individuals were randomly selected using an automated screening procedure programmed in the handheld tablet computers carried by the field interviewers (FIs) or in the web screening questionnaire (see Sections 2.2.1.1 and 2.2.1.3). The number of sample units completing the screening was 90,937.
2.1.1.2 Selection of People within Dwelling Units, by Age Group
As shown in Table 2.2, the allocation of the 2014 through 2022 NSDUH samples is 25 percent for adolescents aged 12 to 17, 25 percent for young adults aged 18 to 25, and 50 percent for adults aged 26 or older. The sample of adults aged 26 or older is further divided into three subgroups: aged 26 to 34 (15 percent), aged 35 to 49 (20 percent), and aged 50 or older (15 percent). Table 2.2 at the end of this chapter provides the target sample allocations for the 2014 through 2022 NSDUHs. Adolescents aged 12 to 17 years and young adults aged 18 to 25 years are oversampled.
2.1.2 Special Changes to the 2020 Sample Design
The sample design included two special changes for the 2020 NSDUH:
- expansion of the sample to support a special clinical validation study (CVS), and
- changes to the sample design in response to the coronavirus disease 2019 (COVID-19) pandemic.
The first of these changes was planned before the start of 2020 NSDUH data collection. The second change was necessitated by the limitations that the COVID-19 pandemic imposed on in-person data collection.
2.1.2.1 Clinical Validation Study Sample
The CVS was originally planned for the first 6 months of the 2020 NSDUH to assess a revised NSDUH section on substance use disorders (SUDs) according to the criteria in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) (American Psychiatric Association [APA], 2013). To support this study, the national sample size of 67,507 interviews was supplemented with 1,500 interviews proportionally allocated to states and age groups in Quarters 1 and 2 (i.e., January to June 2020). Including the supplement, the expanded target sample size for the 2020 NSDUH was 69,007 interviews. From the expanded sample, 1,500 respondents were expected to be selected for the CVS based on their responses to selected questionnaire items (see Table 2.1). Logic in the NSDUH questionnaire then assigned respondents selected for the CVS sample to receive the DSM-5 SUD questions. Table 2.2 shows the target sample allocation for the CVS by age group. Because the CVS supplemental sample was allocated proportionally to the overall NSDUH population, the age group allocation differed from the 2014 through 2022 target samples. As described in Section 2.1.1.2, the sample design for the main survey oversampled adolescents aged 12 to 17 and young adults aged 18 to 25.
All other respondents received the standard set of NSDUH SUD questions that used criteria in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (APA, 1994). Sections 2.2.1.2 and 2.2.2.2 provide more information about the data collection procedures and questionnaire differences for the CVS.
2.1.2.2 Sample Design Changes Because of the COVID-19 Pandemic
Given the public health emergency related to COVID-19 and considering the safety of field staff and the public, NSDUH in-person data collection was suspended on March 16, 2020, including the CVS.11 To assess the feasibility of resuming in-person data collection, a limited sample was fielded for a small-scale data collection from July 16 to 22, 2020, in selected counties within two states where in-person data collection was deemed to pose a low risk of COVID-19 transmission and infection based on state- and county-level health metrics. For the remainder of 2020, the COVID-19 pandemic made it nearly impossible to collect data in person. To mitigate the effect on respondent sample size, web-based data collection was added and Quarter 4 became a period of multimode data collection. For the resumption of data collection in Quarter 4, all sample dwelling units (SDUs) originally selected from the Quarter 2, 3, and 4 area segments for in-person data collection were released for web or in-person data collection. SDUs in the Quarter 2, 3, and 4 area segments were released for in-person data collection in counties where the risk of COVID-19 transmission and infection was deemed to be low beginning on October 1, 2020. SDUs in the remaining Quarter 2, 3, and 4 area segments (except in segments that were worked by FIs during the 1-week, small-scale data collection) were mailed an invitation to participate via the web beginning on October 30, 2020. Additional SDUs also were selected in some segments from Quarters 2 and 3 and released to web-based data collection in Quarter 4 to partially compensate for the negative effect of the COVID-19 pandemic on data collection and response rates. See Section 2.2.1.3 for information on web-based data collection, including the situation in which sampled areas were transferred to web-based data collection because they became ineligible for in-person data collection.
2.1.3 Sample Results for the 2020 NSDUH
In 2020, the actual sample sizes in the 12 largest states12 ranged from 752 to 2,193. In the remaining states, the actual sample sizes ranged from 352 to 723. These sample sizes included the additional respondents for the CVS supplemental sample in Quarter 1. For specific sample sizes by state, see the 2020 sample experience report (Center for Behavioral Health Statistics and Quality [CBHSQ], 2021g).
Adolescents aged 12 to 17 in 2020 were sampled at an actual rate of 81.4 percent, and young adults aged 18 to 25 were sampled at a rate of 70.2 percent on average, when they were present in the sampled households or group quarters. Adults were sampled at rates of 35.5 percent for adults aged 26 to 34, 30.5 percent for adults aged 35 to 49, and 12.4 percent for adults aged 50 or older on average. The overall population sampling rates in 2020 were 0.025 percent for 12- to 17-year-olds, 0.027 percent for 18- to 25-year-olds, 0.015 percent for 26- to 34-year-olds, 0.013 percent for 35- to 49-year-olds, and 0.006 percent for those 50 or older.13 Nationwide, 62,515 individuals were selected. Of these selected individuals, 17,082 completed the interview in person and 19,202 completed the interview via the web. Consistent with previous surveys in this series, the final respondent sample of 36,284 individuals was weighted to be representative of the U.S. civilian, noninstitutionalized population aged 12 or older. In addition, state samples were weighted to be representative of their respective state populations. See Section 2.3.4 for details on weighting. More detailed information on the disposition of the national screening and interview sample can be found in Chapter 3 of this report. More information about the sample design can be found in the 2020 NSDUH sample design report (CBHSQ, 2021f).
2.2 Data Collection Methodology
This section discusses the data collection procedures and questionnaire changes for the 2020 NSDUH.
- Section 2.2.1 discusses in-person data collection procedures (including modifications to in-person data collection procedures in response to the COVID-19 pandemic), data collection procedures for the CVS, and web-based data collection in Quarter 4 (October to December).
- Section 2.2.2 discusses questionnaire changes that were implemented for the entire 2020 data collection period, changes for the CVS, changes in Quarter 4 regardless of whether interviews were completed in person or via the web, and specific changes to accommodate web-based data collection in Quarter 4.
2.2.1 Data Collection Procedures
Data collection methods were modified during 2020 due to the public health emergency related to COVID-19 to ensure the safety of the public and FIs. Quarter 1 (January to March 2020) was completed using standard NSDUH protocols with in-person data collection. However, Quarter 1 data collection ended 15 days early when work was suspended on March 16, 2020. NSDUH project management at RTI began working closely with the Substance Abuse and Mental Health Services Administration (SAMHSA) and RTI’s Infectious Disease Response Team (IDRT), Executive Leadership Team (ELT), and Institutional Review Board (IRB) to determine when and where it would be safe to resume in-person data collection.
To assess the feasibility of resuming in-person data collection, the IDRT advised conducting a small-scale data collection effort. SAMHSA, the ELT, and the IRB approved a 1-week small-scale data collection effort that was conducted from July 16 to 22, 2020. Revisions were made to protocols, processes, and materials to ensure the safety of respondents and FIs. Section 2.2.1.1.2 provides information on revisions to protocols. Analysis of the data and results of a debriefing session with FIs indicated that overall, the safety procedures used during the small-scale data collection effort did not have a negative effect on respondents’ willingness to participate in the survey and FIs were overall pleased with these measures.
Data collection resumed in Quarter 4, but in-person data collection was limited to a small number of eligible states and counties due to COVID-19 infection rates. Web-based screening and interviewing procedures were developed (Section 2.2.1.3) to account for suspended data collection in Quarter 2 and most of Quarter 3 and to maximize the number of completed interviews for national estimates. NSDUH data collection in Quarter 4 used in-person and web-based procedures.
2.2.1.1 In-Person Data Collection
2.2.1.1.1 Standard In-Person Data Collection Procedures
The standard data collection methods used for NSDUH to conduct in-person interviews with sampled individuals incorporate procedures to increase respondents’ cooperation and willingness to report honestly about sensitive topics, such as illicit drug use behavior and mental health issues. Confidentiality is stressed in all written and oral communications with potential respondents. Respondents’ names are not collected with the data, and computer-assisted interviewing (CAI) methods are used to provide a private and confidential setting to complete the interview. Data collection procedures are approved by RTI’s IRB before the start of data collection. Adults aged 18 or older must consent to provide basic data on characteristics of household members (screening) or to complete the main interview. If an adolescent aged 12 to 17 is selected for the main interview, permission from a parent or adult guardian and assent from the selected adolescent must be obtained for the adolescent to complete the interview.
Introductory letters are sent to sampled addresses, followed by an FI visit. FIs make multiple attempts, if necessary, at different days and times to contact the DU. When contacting a DU, the FI asks to speak with an adult resident (aged 18 or older) of the household who can serve as the screening respondent. To obtain basic demographic data on all household members aged 12 or older who lived at the address for most of the calendar quarter, the FI uses a handheld tablet computer to ask the screening respondent a series of questions taking about 5 minutes to complete. The tablet computer then uses the demographic data in a preprogrammed selection algorithm to select zero, one, or two individuals for the interview, depending on the composition of the household. This selection process is designed to provide the necessary sample sizes for the specified population age groupings.
In areas where a third or more of the households contain Spanish-speaking residents, the initial introductory letters written in English are mailed with a Spanish version printed on the back. All FIs carry copies of the introductory letter in English and Spanish. If FIs are not certified bilingual in English and Spanish, they will use preprinted Spanish cards to attempt to find someone in the household who speaks English and who can serve as the screening respondent or who can translate for the screening respondent. If no one is available, the FI’s field supervisor will schedule a time when a certified bilingual FI can come to the address. In households where a language other than Spanish is encountered, another language card is used to attempt to find someone who speaks English to complete the screening.
The NSDUH interview can be completed in English or Spanish, and both versions have the same content. If the sampled person prefers to complete the interview in Spanish, a certified bilingual FI is sent to the address to conduct the interview. Because the interview is not translated into any other language, if a sampled person does not speak English or Spanish, the interview is not conducted.
Immediately after completion of the screener, FIs attempt to obtain consent and conduct the NSDUH interview with each sampled person in the household. The FI asks the respondent to identify a private area in the home in which to conduct the interview away from other household members. The FI uses a laptop computer to conduct the interview, which averages about an hour and includes a combination of computer-assisted personal interviewing (CAPI) and audio computer-assisted self-interviewing (ACASI). In the CAPI portion of the interview, the FI reads the questions to the respondent and enters the answers into the computer. In the ACASI portion of the interview, the respondent reads questions on the computer screen or listens to questions through headphones, then keys in answers directly into the computer without the FI knowing the response.
The NSDUH in-person interviews begin in the CAPI mode and consist of initial demographic questions. The interview then transitions to the ACASI mode for the sensitive questions (e.g., use of tobacco, alcohol, illicit drugs14). Additional self-administered interview sections15 follow the substance use questions and ask about a variety of sensitive topics (e.g., injection drug use, SUDs, substance use treatment, mental health issues, use of mental health services, important emerging substance use and mental health issues, perceived effects of the COVID-19 pandemic).
Although many of the questions about mental health issues are asked both of youths aged 12 to 17 and of adults, some are asked only of adults, and others are asked only of youths. Definitions for many of the terms for substance use and mental health measures from the survey are included in the glossary in Appendix A of this report.
Additional demographic questions addressing topics such as immigration, current school enrollment, and employment and workplace issues are included at the end of the ACASI section for in-person interviews. Finally, the in-person interviews return to the CAPI administration for questions on the household composition, the respondent’s health insurance coverage, and the respondent’s personal and family income. Each respondent who takes the time to complete a full interview is given a $30 cash incentive as a token of appreciation.
No information directly identifying a respondent is captured in the in-person CAI record. FIs transmit completed interview data to RTI in Research Triangle Park, North Carolina. Screening and interview data are encrypted while they reside on the laptop and tablet computers. Data are transmitted back to RTI on a regular basis using a wireless connection to the Internet. All data are encrypted while in transit across Internet connections. In addition, in-person screening and interview data are transmitted back to RTI in separate data streams and are kept physically separate (on different devices) before transmission occurs.
After in-person data are transmitted to RTI, certain respondent records are selected for verification. Respondents are contacted by RTI to verify the quality of an FI’s work based on information respondents provide at the end of screening (e.g., if no one is selected for an interview or all household members at the sampled address are ineligible for the study) or at the end of the interview. For the screening, adult household members who served as screening respondents provide their first names and telephone numbers to FIs who enter the information into tablet computers and transmit the data to RTI. For completed interviews, respondents write their telephone numbers and mailing addresses on quality control forms and seal the forms in preaddressed envelopes FIs mail back to RTI. All contact information is kept completely separate from the answers provided during the screening or interview.
Samples of respondents who completed screenings or interviews are randomly selected for verification. These respondents are called by telephone interviewers who ask scripted questions designed to determine the accuracy and quality of the data collected. Any sampled screening or interview discovered to have a problem or discrepancy is flagged and routed to a small specialized team of telephone interviewers who recontact respondents for further investigation of the issue(s). Depending on the amount of an FI’s work that cannot be verified through telephone verification, including bad telephone numbers (e.g., incorrect number, disconnected, not in service), a field verification may be conducted. Field verification involves another FI returning in person to the sampled address to verify the accuracy and quality of the data. If the verification procedures identify situations in which an FI falsified data, the FI is terminated from employment. All screenings or interviews completed that quarter by the falsifying FI are verified by the FI conducting the field verification and are reworked.
2.2.1.1.2 Modifications to In-Person Data Collection Procedures Because of the COVID-19 Pandemic
In response to the COVID-19 pandemic, RTI’s IDRT developed eligibility criteria for in-person data collection based on COVID-19 infection rates and state government mandates, such as stay-at-home orders. The criteria were based on state- and county-level infection rates. The 2020 data collection final report (CBHSQ, forthcoming b) will provide details on the specific state- and county-level criteria that were applied to in-person data collection in July 2020 and again from October 1 to December 31, 2020.
The general procedures for in-person data collection described in Section 2.2.1.1.1 typically applied when in-person data collection resumed, including procedures for transmitting completed interviews to RTI and for verification. However, revisions needed to be made to in-person protocols, processes, and materials to protect the health and safety of respondents and FIs. The revisions included providing FIs with the following: (1) RTI-issued masks, gloves, disinfecting wipes, and hand sanitizer for use during data collection; (2) a NSDUH Safety Protocol Reference Guide that outlined all required safety procedures; and (3) a risk information form provided to all respondents. Examples of the safety protocol reference guide and the risk information form will be provided in the 2020 data collection final report (CBHSQ, forthcoming b). These revised procedures were used in the 1-week small-scale data collection effort in July 2020 and during Quarter 4.
During the 1-week in-person data collection in July 2020, NSDUH FIs who approached a DU to conduct a screening were required to wear a disposable face mask and keep 6 feet of distance between themselves and others when possible. FIs were asked to encourage respondents (or potential respondents) to wear a mask, but respondents were not required to do so. After a brief introduction and confirmation that the address was correct, FIs completed the informed consent procedures for the screening and handed each respondent a study description. FIs placed all materials to be handed to respondents in a plastic sleeve 3 days prior to use. For Quarter 4, this procedure was updated to require FIs to place materials in separate folders 3 days prior to use: one folder for each DU screening and separate folders for each interview respondent.
In both July 2020 and Quarter 4, a new requirement for informed consent due to COVID-19 was to provide each respondent with a printed copy of the COVID-19 risk information form to keep. FIs and respondents reviewed the content of this form together to be sure each potential respondent was aware of the risks associated with participation in NSDUH and to allow each person to make an informed decision about participating in the study due to the potential risks of COVID-19 transmission and infection.
If a DU member was selected to complete the interview, the FI was encouraged, when possible, to recommend conducting the interview outside of the home, such as on a porch, deck, or patio. This procedure was recommended so the FI would avoid entering people’s homes for a prolonged period of time. While setting up the laptop computer for the interview after the respondent had consented to participate, the FI, in the presence of the respondent, used disinfectant wipes to clean the surface of the laptop, including the keyboard, the headphones, and the Showcard Booklet.
Unlike regular in-person data collection, FIs did not attempt follow-up refusal conversion. Records were assigned a final code as a refusal for screening or interview immediately if a DU member mentioned COVID-19 as a concern. If there was no contact with anyone in the household, in-person attempts were limited to 10 visits. In addition, revisions were made to the NSDUH FI authorization letter that FIs carry with them in the field to designate FIs as “essential workers.”
2.2.1.2 Clinical Validation Study Data Collection
During the first 3 months16 of the 2020 NSDUH main study data collection, the CVS was also conducted to assess a revised SUD module developed by SAMHSA for inclusion in the NSDUH main study questionnaire. Sections 2.2.2.2 and 3.4.3.4 provide more information on the development and content of the CVS questions.
Respondents were eligible for the CVS sample if they chose to answer the NSDUH main study interview questions in English17 and did not break off the interview before beginning the SUD module.18 The CVS sample was selected by the NSDUH CAI instrument. Based on their responses to questions about past year use of cigarettes, alcohol, and marijuana, respondents selected for the CVS were routed to the new DSM-5 SUD module instead of the DSM-IV module. Otherwise, CVS sample members completed the main study modules in the same order as the non-CVS sample.19
At the end of the NSDUH main study interview, CVS sample respondents were invited by the FIs to participate in a follow-up clinical interview. NSDUH main study respondents who agreed to participate in the follow-up interview and provided relevant contact information were part of the CVS follow-up sample.
The follow-up clinical interviews were completed via telephone within 2 to 4 weeks of the NSDUH main study interview. The follow-up interviews were conducted using a modified paper version of the Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV) (First et al., 2015). The SCID is a semi-structured diagnostic interview used to assess psychiatric disorders according to the criteria in DSM-5. As a semi-structured clinical interview, the SCID contains structured, standardized questions that are read verbatim and sequentially, combined with unstructured follow-up questions that the clinical interviewer tailors to the respondent based on clinical judgment and the respondent’s answers. The SCID was administered over the telephone by clinical interviewers who underwent 4 days of extensive training with clinical supervisors and Dr. Michael First, the SCID’s developer from Columbia University. Within 48 hours after completing an interview, the clinical interviewer edited and shipped the paper SCID to RTI for final editing and keying in-house.
2.2.1.3 Web-Based Data Collection
As noted previously, SAMHSA decided to suspend NSDUH in-person data collection on March 16, 2020, due to the COVID-19 public health emergency. To facilitate data collection while minimizing risks to respondents and FIs, SAMHSA approved the addition of web-based data collection starting in Quarter 4 (October to December 2020).
Web-based data collection in NSDUH followed the same basic steps as in-person data collection, but the procedures were modified for the web environment:
- making contact with the SDU;
- screening of the SDU to identify residents aged 12 or older and determining whether zero, one, or two members would be selected to complete the interview;
- obtaining consent from SDU members aged 18 or older or parental permission and respondent assent from youths aged 12 to 17 who were selected for an interview; and
- administering the NSDUH questionnaire to consenting respondents.
Details about the web-based screening and interviewing procedures, as well as the key differences compared with in-person data collection, will be provided in the 2020 data collection final report (CBHSQ, forthcoming b). Implications of the multimode data collection for 2020 NSDUH estimates are discussed in Chapter 6.
An important distinction between in-person and web-based data collection was that responsibility rested on members of SDUs to keep the data collection process moving forward to the next stages. For example, no web-based data collection occurred if SDU members did not respond to invitation letters or follow-up letters (see below). For in-person data collection, the practice was for FIs to contact SDUs regardless of whether SDU members read the introductory letter.
Also, because FIs were not present to assist SDU members with questions, an important feature of web-based data collection also was the availability of a “Contact NSDUH” link (along with a toll-free number) for technical support and answers to questions about participation in NSDUH.
As for in-person data collection, confidentiality was stressed in communications with potential web respondents. Respondents’ names were not collected with the interview data. Procedures for web-based data collection procedures were approved by RTI’s IRB before the start of data collection. The website’s https encryption provided sufficient security for information entered from devices (e.g., smartphone, tablet, computer) via any Internet connection (e.g., public Wi-Fi, cellular, at-home Wi-Fi).
Introductory letters were sent to mailable addresses20 that were selected for web-based data collection. The content of letters depended on whether SDUs had been selected for web-based data collection at the outset in Quarter 4 or whether SDUs were located in counties that started out as being eligible for in-person data collection but later became ineligible because of increased COVID-19 infection rates. Introductory letters for web-based data collection provided the website address to access the online screening and a unique participant code specific to each SDU that was needed to log in to complete screening and interviewing via the web. Web lead letters also included details about NSDUH and contained the address to the NSDUH respondent website for general information about the study and the toll-free number for the NSDUH respondent call line.
SDUs with pending screenings or interviews that subsequently became ineligible due to the COVID-19 pandemic for in-person data collection became eligible for web-based data collection. Further information on these SDUs with pending screenings or interviews will be provided in the 2020 data collection final report (CBHSQ, forthcoming b). Although SDUs could transition from in-person to web-based data collection, SDUs were not permitted to transition from web-based to in-person data collection for the remainder of 2020, regardless of decreases in COVID-19 infection rates in the counties where those SDUs were located. If an SDU transferred from in-person to web-based data collection, the SDU received a web introductory letter in the mail notifying SDU members of this change and providing the website address and participant code to access the web screening. The type of letter received at the SDU as part of this transition to web-based data collection depended on the status of the in-person data collection, such as if screening had been completed but there were pending interviews.
SDUs received follow-up letters that contained the relevant information for web participation if screening or interviewing was not completed. People from a selected SDU who called the respondent call line and stated they did not wish to participate were noted as refusals in the project database so that they stopped receiving follow-up correspondence.
Like in-person data collection, screening respondents needed to be at least 18 years old and interview respondents needed to be at least 12 years old for web-based data collection. Unlike in-person data collection, screening and interview respondents for web-based data collection also needed to be able to read English or Spanish to participate. For screenings, FIs were not available to assist web respondents, and, therefore, there may be mode differences in survey items due to respondent understanding. Also different from in-person data collection, for the main interview, respondents with limited visual ability or reading skills did not have access to audio recordings of the questions.21 Therefore, SDU members who were blind or unable to read English or Spanish were not eligible to be web-based screening or interview respondents. SDU members who did not have Internet access or access to an Internet-compatible device (e.g., smartphone, tablet, computer) also were ineligible to be screening or interview respondents via the web.
An adult resident of the SDU who chose to participate could access the NSDUH web screening instrument from any device with Internet access (e.g., smartphone, tablet, computer). As noted previously, adult residents needed to enter the unique participant code found in the web introductory letter to access the screening interview. Unlike in-person interviews, the consent process for screening involved adult SDU members reading the consent information on-screen and recording their consent online before screening could proceed. However, adults could call toll-free numbers to ask questions before consenting to screen the SDU.
Like the in-person screening, the web screening questions collected basic demographic information for all SDU members aged 12 or older for the web screening program to determine whether zero, one, or two residents of the SDU aged 12 or older were selected for the NSDUH web interview. The screening concluded if no members of the SDU were selected for an interview. If one or two SDU members were selected for an interview, these SDU members were identified on the interview selections screen according to their age and relationship to the screening respondent (e.g., 14-year-old son, 46-year-old wife) rather than by name.
If the screening respondent was selected for the main interview, the process could move forward with obtaining consent and completing the main interview. If SDU members other than the screening respondent were selected for the main interview, “handoff” responsibility rested with the screening respondent to notify other SDU members of their selection. The consent procedures for adults also applied if an adult other than the screening respondent was selected for the interview.
If one or both of the SDU members selected for an interview were youths aged 12 to 17, verbal parental permission and youth assent were required via telephone before the youth could participate in the interview. Using a toll-free number, the parent and the youth were required to call together to speak with an RTI project representative before proceeding with the interview. Once parental permission and youth assent were given verbally, the project representative recorded in the project database that parental permission and youth assent were provided for the interview. At that point, the youth interview could be accessed from the NSDUH website.
Once interview respondents used the participant code to access their assigned interview, they could choose to complete the interview in either English or Spanish. Interview respondents were encouraged to complete the interview in one sitting. Respondents were advised that they would be automatically logged out of the interview after 15 minutes of inactivity and that after 60 minutes of inactivity, all previously entered responses would be deleted for security purposes. Interview respondents also could access their interview at a later time of their choosing (until the end of the data collection period in December) by using the website address—the same address for the screening—and inserting the participant code unique to that SDU.
If SDU members did not complete the web-based interview immediately following the completion of the web screening, a follow-up letter was mailed to the sampled SDU member, addressed by age and gender (e.g., 46-year-old female resident, parent of 14-year-old male resident), after the screening was completed. If parental permission and youth assent were not obtained from sampled youths immediately following the web screening, a follow-up letter was mailed to the sampled youth’s parent(s) after the screening was completed to notify the parents of the parental permission requirement and procedures for the youths to complete the interview. Follow-up letters (regardless of SDU member age) were mailed to the SDUs once each week for 3 weeks from the day the screening was completed.
Respondents for the web-based interview, whether adults or youths, were asked to be in a private location within the home and to affirm before starting an interview that they were in a private location. At multiple points during the interview—especially before particularly sensitive sections of the interview—respondents were reminded that they should be in a private location.
As an additional layer of security, each respondent was required to set a unique 4-digit PIN code of their choosing to prevent anyone else within the dwelling unit from accessing the interview and seeing answers to questions. Because no one at RTI had access to these PIN codes, however, there was no way to assist respondents who forgot their PIN.
Every interview respondent who completed the web-based interview selected a preferred method for receiving a $30 incentive, either an electronic Visa or MasterCard gift code sent to the relevant email address of choice or a physical Visa or MasterCard gift card delivered to the SDU. Information for delivery of the incentive was kept separate from interview responses. Web-based screening and interview data that were received at RTI were stored in a heightened security network that required two forms of authentication for access.
Unlike in-person data collection, completed web interviews that were selected for verification came directly from respondents rather than from FIs. To ensure that SDU members who were selected to complete the main interview were the actual respondents who provided data, completed interview data were monitored for internal consistency to verify that SDU roster demographics reported during the screening matched those reported during the interview. Completed interviews also were monitored for situations where the self-reported age during the interview differed from the sample member’s reported age during the screening. Interviews that appeared to have been completed by someone other than the selected SDU member were removed from the dataset.
2.2.2 Notable Questionnaire Changes for 2020
2.2.2.1 Changes for the Entire Data Collection Period
Notable changes for the 2020 questionnaire that were available for the entire data collection period included the following:
- A new emerging issues section was created that included questions from the 2019 survey and new questions on specific topics, such as new topics on vaping (for any substance and nicotine), synthetic cannabinoid use, and synthetic cathinone use.
- The section included existing questions from the 2019 survey on the following topics: perceived recovery, receipt of medication-assisted substance use treatment, and kratom use.
- New questions were added for the lifetime and most recent vaping of any substance and vaping of nicotine or tobacco.
- New questions were added for the lifetime and most recent use of synthetic cannabinoids (referred to in the questionnaire for simplicity as “synthetic marijuana,” with the slang terms “fake weed,” “K2,” and “Spice”).
- New questions were added for the lifetime and most recent use of synthetic cathinones (referred to in the questionnaire for simplicity as “synthetic stimulants,” with the slang terms “bath salts” and “flakka”).
- New questions were added to measure SUD symptoms in the DSM-5 for marijuana withdrawal, prescription tranquilizer misuse withdrawal, and the symptom of craving (i.e., a strong desire or urge to use) for all substances.
- In the market information for marijuana section:
- A new question was added about purchasing marijuana from a store or dispensary.
- Respondents who reported that they last purchased marijuana from a store or dispensary were skipped out of questions for other specific settings where they purchased marijuana.
2.2.2.2 Clinical Validation Study Questions in Quarter 1
The CVS was embedded within the first quarter of 2020 NSDUH data collection to assess SUD questions that were revised to be consistent with the DSM-5 criteria for SUD. NSDUH respondents in Quarter 1 (January to March 2020) who answered the survey in English and reported using alcohol or illicit drugs in the past 12 months were randomly assigned to be asked revised SUD questions based on the DSM-5 criteria or the standard NSDUH SUD questions based on the DSM-IV criteria. Respondents who received the DSM-IV SUD questions also were eligible to receive questions in the emerging issues section of the interview for marijuana withdrawal symptoms, prescription tranquilizer withdrawal symptoms, and craving for all substances they used or misused in the past year, as described in Section 2.2.2.1. These additional symptoms applied to the DSM-5 SUD criteria but were not measured in the existing DSM-IV SUD questions.
2.2.2.3 Questionnaire Changes for Quarter 4, Regardless of Interview Mode
Several key questionnaire changes were made for the resumption of data collection in Quarter 4. Unless noted otherwise, these changes were made for both the in-person and web-based questionnaires. Key changes that needed to be made for web administration are described in Section 2.2.2.4.
- The Beginning ACASI section of the in-person interview, which guides the FI through the process of explaining important laptop keys and specific interview program functions to the respondent, was redesigned to be self-administered to minimize the risk of COVID-19 transmission. This modification allowed the FI to pass the laptop computer to a respondent and maintain social distancing without sacrificing necessary instructions.
- The DSM-5 SUD questions that were administered for the CVS in Quarter 1 were removed for Quarter 4 data collection in the in-person and web questionnaires due to the closure of the study. (Additional DSM-5 questions remained in the emerging issues section for Quarter 4.)
- Two questions were added to the drug treatment section to measure the use of telehealth services for alcohol or drug use issues in the past 12 months.
- A question was added to the health section to measure the use of telehealth services for health care in the past 12 months. Respondents who reported telehealth service use in the past 12 months were eligible to be asked subsequent questions in the health section that asked whether health care providers obtained information about substance use (i.e., the use of tobacco, alcohol, or specific illicit drugs) or offered health care advice related to respondents’ substance use.
- A question was added to the adult mental health service utilization section and to the youth mental health service utilization section to measure use of telehealth services for mental health or behavioral services in the past 12 months.
- All adult respondents received questions about suicide plans or attempts in the past 12 months, regardless of whether they reported having serious thoughts of suicide in the past 12 months. (In Quarter 1 and in prior years, respondents needed to report serious thoughts of suicide to be asked questions about suicide plans or attempts.) See Sections 3.4.16.1 and 6.2.3 for more details.
- Follow-up questions were added after each adult suicidality item in the mental health section if respondents reported serious thoughts of suicide, suicide plans, or suicide attempts in the past 12 months. These follow-up questions asked whether these thoughts of suicide, suicide plans, or suicide attempts were because of the COVID-19 pandemic.
- Suicide items were added for youths in the youth mental health service utilization section.
- These items mirrored the adult suicide items in Quarter 4, including the new COVID-19 follow-up questions.
- An additional text screen was provided for youths who reported serious thoughts of suicide, suicide plans, or suicide attempts. This text screen provided information on how to contact the National Lifeline Network.
- A series of self-administered questions related to the COVID-19 pandemic were added toward the end of the interview for adults and youths. These questions asked about respondents’ perceptions of effects of the COVID-19 pandemic on their mental health, substance use, finances, living situation, and access to services.
- For the in-person interview, instructions were changed at the end of the interview to limit contact between the FI and respondent during the quality control and incentive procedures.
2.2.2.4 Questionnaire Changes for Quarter 4 Web Interviews
Far-reaching questionnaire changes were necessary for web administration. The following list includes key changes to facilitate web administration and differences between the web version of the survey and the in-person interview.
- A variable that listed whether the selected respondent was an adult or a youth was “preloaded” to the interview so that the correct informed consent information could be displayed at the beginning of the interview.
- Information about the respondent’s state of residence and sample information from the household screener (i.e., whether two people were selected at the DU and whether an adolescent aged 12 to 17 was selected) was “preloaded” because there were no FIs to enter the information.
- Information previously entered by the FI, such as language of the interview and the informed consent process, were adapted to be self-administered.
- A PIN creation process was added after the informed consent process, but before any questions, to safeguard respondent data and allow respondents to reenter the interview at a later time.
- Sections that were interviewer administered using CAPI in the in-person interviews were modified for self-administration. For example, references to showcards in the in-person CAPI sections were dropped from the web interview because those did not apply for self-administration.
- For DUs in which two people were selected, one of them was aged 12 to 17, and the second person was an adult, eligibility for the parenting experiences section was determined by asking at the start of the parenting experiences section whether the adult respondent was the parent of the 12- to 17-year-old who was selected. In the in-person interviews, this information was entered by FIs at the beginning of the interview.
- Hard errors and consistency checks were simplified throughout the interview to avoid respondent confusion and frustration.
- The web questionnaire did not include audio. Instead, pronunciations were spelled out visually on several screens, particularly for hallucinogens, inhalants, and prescription drug introduction screens, to help youths and respondents with a lower reading level understand the questions accurately.
- On-screen interviewer notes were either removed from the web questionnaire or adapted for self-administration.
2.3 Data Processing
Survey data received at RTI, either transmitted from FIs for in-person interviews or captured directly from the web-based data collection, are processed to create a raw data file in which no logical editing of the data has been done. The raw data file consists of one record for each interview. Interview records are eligible to be treated as final respondents only if people provided data on lifetime use of cigarettes and at least 9 out of 13 of the other substances in the initial set of substance use questions described in Section 2.3.1. Even though editing and consistency checks are done by the CAI program during the interview, additional, more complex edits and consistency checks are completed at RTI. Also, statistical imputation is used to replace missing, inexact, or nonspecific values after editing for some key variables. Analysis weights are created so that estimates will be representative of the target population.
2.3.1 Criteria for Identifying Usable Interviews
A key step in the preliminary data processing procedures establishes the minimum item response requirements for interviews to be used in weighting and further analysis (i.e., “usable” data). These procedures are designed to disregard data from interviews with unacceptable levels of missing data at the outset, thereby using data from interviews with lower levels of missing data and reducing the amount of statistical imputation needed for any given record.
The following criteria were used beginning with the 2015 NSDUH to establish whether interview data could be considered usable:
- The lifetime cigarette gate question CG01 must be answered as “yes” or “no.”22
- In addition to the criterion for cigarettes, “usability” must be determined for at least nine (9) of the following other substances: (a) smokeless tobacco, (b) cigars, (c) alcohol, (d) marijuana, (e) cocaine (in any form), (f) heroin, (g) hallucinogens, (h) inhalants, (i) methamphetamine, (j) prescription pain relievers, (k) prescription tranquilizers, (l) prescription stimulants (i.e., independent of methamphetamine), and (m) prescription sedatives.
Crack cocaine was not included in the usability criteria because the logic for asking about crack cocaine was dependent on the respondent having answered the lifetime cocaine question as “yes.” Although NSDUH respondents were also asked about pipe tobacco, this tobacco product was not included in the usability criteria because there was only one other question about pipe tobacco in addition to the lifetime pipe tobacco use question. For the “multiple gate” sections for hallucinogens and inhalants, at least one gate question in the series for that section was required to have an answer of “yes” or “no.” Any of the following allowed the prescription drug data to count toward usability:
- past year use of at least one specific prescription drug in a category (e.g., pain relievers) is reported;
- lifetime use or nonuse of any prescription drug in the category is reported; or
- past year nonuse of all specific prescription drugs23 is reported, regardless of whether lifetime use or nonuse can be determined.24
2.3.2 Data Coding and Editing
The data coding and logical editing procedures discussed below applied to all respondents for 2020. The same procedures were followed regardless of whether data were collected in person (including the CVS in Quarter 1) or through the web.
Coding of answers to open-ended questions typed by respondents or FIs (the latter only for in-person data) was performed at RTI for the 2020 NSDUH. Because these open-ended questions typically include the word “other” (e.g., whether respondents ever used “any other hallucinogens,” whether respondents received treatment for their use of alcohol or other drugs in “some other place” in the past 12 months), data from these questions are subsequently referred to as “OTHER, Specify” data. For example, if respondents reported that they ever “used any other hallucinogens besides the ones that have been listed,” they subsequently could specify the names of up to five other hallucinogens that they used.
Written responses in “OTHER, Specify” data were assigned numeric codes through computer-assisted survey procedures and the use of a secure website allowing for coding and review of the data. The computer-assisted procedures entailed a database check for a given “OTHER, Specify” variable containing typed entries and the associated numeric codes. If an exact match was found between the typed response and an entry in the system, then the computer-assisted procedures assigned the appropriate numeric code. Typed responses not matching an existing entry were coded through the web-based coding system.
Elsewhere in the interview, the CAI program included checks to alert respondents or FIs (the latter only for in-person data) when they entered an answer that was inconsistent with a previous answer. For example, respondents could report that the last time they used Ecstasy was more recent than the last time they used any hallucinogen; these data triggered a consistency check to alert respondents to the inconsistency. In this way, the inconsistency could be resolved while the interview was in progress. However, not every inconsistency was resolved during the interview even if respondents were alerted to the inconsistency. For example, respondents could continue to report that their last use of Ecstasy was more recent than their last use of any hallucinogen despite being given the opportunity to resolve the inconsistency. In this situation, the inconsistency was resolved through logical editing by inferring a response for the most recent use of any hallucinogen that was consistent with the final answer for the most recent use of Ecstasy. In addition, the CAI program did not include checks for every possible inconsistency that might have occurred in the data.
Therefore, the first step in processing the raw NSDUH data was logical editing of the data. Logical editing involved using data from within a respondent’s record to (1) reduce the amount of item nonresponse (i.e., missing data) in interview records, including identification of items legitimately skipped; (2) make related data elements consistent with each other; and (3) identify inexact, nonspecific, or inconsistent responses needing to be resolved through statistical imputation procedures (see Section 2.3.3). See the 2019 NSDUH editing and imputation report (CBHSQ, 2021b) for the most recent documentation of editing procedures.
2.3.2.1 General Principles of Editing NSDUH Data
Because the CAI logic controlled whether respondents were asked certain questions based on their answers to previous questions, an important aspect of editing the NSDUH data involved identifying where questions had been legitimately skipped because they did not apply, as noted above. Examples where questions were legitimately skipped include situations in which questions applied to (1) an event (e.g., use of a particular substance) occurring at least once in the respondent’s lifetime, but the respondent previously reported the event never occurred; (2) an event occurring in a particular time period (e.g., within the past 12 months), but the respondent previously reported the event occurred less recently; or (3) respondents with a particular demographic characteristic (e.g., adults aged 18 or older), but the respondent was not part of that group. These scenarios are represented by different codes in the edited variables.
Another important principle in editing the data was that responses from one section (e.g., pain relievers) generally were not used to edit variables in another section (e.g., tranquilizers). For example, if a respondent specified the misuse of a tranquilizer as some other pain reliever the respondent misused in the past 12 months, then this “OTHER, Specify” response for pain relievers was not used to edit the data for tranquilizers. This principle of not using data in later sections to edit data in earlier sections has been important for maintaining consistent data to assess trends in outcomes of interest (e.g., substance use).25
One exception to this principle of not editing across sections involved situations in which responses in one or more sections governed whether respondents were asked questions in a later section. For example, the substance use treatment section was relevant only for respondents who reported some lifetime use or misuse of alcohol or other drugs, excluding tobacco products. Respondents who reported in the initial substance use sections they had never used alcohol or illicit drugs were not asked the questions in the substance use treatment section. In this situation, the responses from the earlier substance use sections were used to edit the substance use treatment variables to indicate respondents were not asked the substance use treatment questions because they reported they never used or misused any of the relevant substances.
2.3.2.2 Editing of Data for Tobacco through Methamphetamine
In sections of the interview for tobacco, alcohol, marijuana, cocaine (including crack cocaine), heroin, and methamphetamine, respondents were asked single questions about lifetime use or nonuse. In the hallucinogens and inhalants sections, respondents were asked a series of questions about lifetime use or nonuse of specific substances in these categories (e.g., “LSD, also called ‘acid’” as a specific hallucinogen). If respondents reported they never used a given substance, either in the single lifetime question or in the series of specific lifetime questions (depending on the substance), the CAI logic skipped them out of all remaining questions about use of that substance. In the editing procedures, the skipped variables were assigned specific codes to indicate the respondents were lifetime nonusers.
In addition, respondents could report they were lifetime users of a drug but not provide specific information on when they last used it. In this situation, a temporary “indefinite” value for the most recent period of use was assigned to the edited recency-of-use variable (e.g., “Used at some point in the lifetime LOGICALLY ASSIGNED”), and a final, specific value was statistically imputed. The editing procedures for key drug use variables also involved identifying inconsistencies between related variables so that these inconsistencies could be resolved through statistical imputation. For example, if respondents reported last using a drug more than 12 months ago and also reported first using it at their current age, both of those responses could not be true. In this example, the inconsistent period of most recent use was replaced with an “indefinite” value, and the inconsistent age at first use was replaced with a missing data code. These indefinite or missing values were subsequently imputed through statistical procedures to yield consistent data for the related measures, as discussed in Section 2.3.3.
2.3.2.3 Editing of Prescription Drug Data
In the prescription drug questionnaire sections, respondents first were asked a series of screening questions about any use of specific prescription drugs in the past 12 months (i.e., use or misuse) or any lifetime use if they did not report past year use. Respondents were then asked about misuse in the past year of any of the specific prescription drugs they reported using in that period.
Consistent with the general editing principles, an important component of editing the prescription drug variables in 2020 involved assignment of codes to indicate when respondents were not asked inapplicable questions. For example, if respondents did not report use of a particular drug in the past 12 months, then the corresponding edited variables for misuse of that drug in the past 12 months were assigned codes to indicate the questions did not apply.
Because of the structure of the prescription drug questions, respondents were not asked a specific question for their most recent misuse of any prescription drug in that general category (e.g., most recent misuse of any pain reliever). Rather, variables for the most recent misuse of prescription pain relievers, tranquilizers, stimulants, and sedatives were created from respondents’ answers to questions about the misuse of any prescription drug in the category in the past 30 days, misuse of specific prescription drugs in a given category in the past 12 months, and lifetime misuse of any prescription drug in the category. The following general principles were applied in creating the variables for the most recent misuse of any prescription drug in a given category in the 2020 data:
- Respondents who reported misuse of prescription drugs in the past 30 days were classified as having last misused prescription drugs in the past 30 days.
- Respondents who reported misuse of one or more specific prescription drugs in the past 12 months were classified as having last misused prescription drugs more than 30 days ago but within the past 12 months, provided they reported they did not misuse any drug in that category in the past 30 days.
- Respondents who reported lifetime (but not past year) misuse of prescription drugs were classified as having last misused prescription drugs more than 12 months ago, provided (1) they answered all applicable questions about misuse of specific prescription drugs in the past 12 months as “no”; or (2) they reported any use of prescription drugs in their lifetime and they explicitly reported they did not use any prescription drug in that category in the past 12 months.
- Respondents who reported they never used or never misused prescription drugs were classified as never having misused prescription drugs. (The coding of the variables for most recent use did not distinguish between respondents who never used prescription drugs and lifetime users who never misused prescription drugs.)
As for the substances discussed in Section 2.3.2.2, some respondents provided indefinite information on when they last misused prescription drugs. For example, if respondents reported misuse of one or more specific prescription drugs in the past 12 months but they did not know or refused to report whether they misused any prescription drug in the past 30 days, it could be inferred these respondents misused prescription drugs in the past 12 months and potentially in the past 30 days. In these situations, a temporary “indefinite” value for the most recent period of misuse was assigned to the variables created for the most recent misuse of pain relievers, tranquilizers, stimulants, and sedatives (e.g., “Used at some point in the past 12 months LOGICALLY ASSIGNED”), and a final, specific value was statistically imputed.
In addition, respondents were instructed in the prescription drug sections not to report the use or misuse of over-the-counter drugs. Therefore, if a respondent’s only report of misuse in the past 12 months was for an over-the-counter drug, the respondent was logically inferred not to have misused any prescription drug in that category in the past 12 months. These respondents were not asked about lifetime misuse of any prescription drug in that category because the CAI program handled them as though they had misused prescription drugs in the past 12 months. Consequently, statistical imputation was used to assign a final value for whether these respondents misused prescription drugs more than 12 months ago or never in their lifetime.
2.3.2.4 Editing of Mental Health Data
An important aspect of editing the mental health variables was documentation of situations in which it was known unambiguously that respondents legitimately skipped out of the corresponding questions. These included situations in which respondents were not asked questions based on their age and those based on routing logic within a given set of mental health questions. For example, if adult respondents reported they did not stay overnight or longer in a hospital or other facility to receive mental health services in the past 12 months, the CAI logic skipped them out of all remaining adult mental health treatment utilization questions about inpatient mental health services. In the editing procedures, the skipped variables were assigned codes to indicate these additional inpatient adult mental health services variables did not apply.
As noted in Section 2.2.2.3, all adult respondents beginning in Quarter 4 (i.e., beginning in October 2020) received questions about suicide plans or attempts in the past 12 months, regardless of whether they reported having serious thoughts of suicide in the past 12 months. Before Quarter 4 and in prior years, respondents needed to report serious thoughts of suicide to be asked questions about suicide plans or attempts. Consequently, more adult respondents in Quarter 4 were asked questions about suicide plans and attempts in the past 12 months. Therefore, two sets of edited variables were created for 2020 for the affected suicide measures. The first set was defined for respondents interviewed prior to October 2020 and retained the skip logic before the questionnaire change in Quarter 4. The second set was defined for respondents interviewed in Quarter 4 (i.e., October to December 2020) and took into account the new skip logic. The second set of edited variables will be used in future years because this change in Quarter 4 of 2020 will apply to all adult respondents for the 2021 NSDUH.
2.3.3 Statistical Imputation
Imputation is defined as the replacement of missing values with substituted values. For a subset of NSDUH variables, missing data are replaced with statistically imputed data after editing is complete. This section provides an overview of the statistical imputation procedures implemented for the 2020 NSDUH. Section 2.3.3.1 discusses the general approach to imputation. Section 2.3.3.2 discusses modifications to the imputation procedures to account for special issues in 2020.
2.3.3.1 General Imputation Approach
For substance use, SUD, demographic, and other key variables still having missing or nonspecific values after editing, statistical imputation was used to replace these values with appropriate response codes. The mental health variables related to mental health service utilization, suicidal thoughts and behavior, and major depressive episode used in reports and tables were not imputed. See Sections 3.4.7.4 and 3.4.7.5 for discussion of how missing data were handled for variables for psychological distress and impairment due to psychological distress among adults. These variables were important for prediction of any mental illness and serious mental illness in the past year among adults.
The remainder of this section discusses procedures for substance use and other variables that underwent statistical imputation to replace missing or nonspecific values. For example, a response is nonspecific if the editing procedures assigned a respondent’s most recent use of a drug to “Used at some point in the lifetime,” with no definite period within the lifetime. In this situation, the imputation procedure assigns a value for when the respondent last used the drug (e.g., in the past 30 days, more than 30 days ago but within the past 12 months, more than 12 months ago). Similarly, if a response is completely missing, the imputation procedures replace missing values with nonmissing ones. See the 2019 NSDUH editing and imputation report (CBHSQ, 2021b) for an overview of the general imputation process.
For most variables, missing or nonspecific values are imputed in NSDUH using a methodology called predictive mean neighborhood (PMN), which was developed specifically for the 1999 survey and has been used in all subsequent survey years. PMN allows for the following: (1) the ability to use covariates to determine donors is greater than that offered in the hot-deck imputation procedure, (2) the relative importance of covariates can be determined by standard modeling techniques, (3) the correlations across response variables can be accounted for by making the imputation multivariate, and (4) sampling weights can be easily incorporated in the models. The PMN method has some similarity with the predictive mean matching method of Rubin (1986) except, for the donor records, Rubin used the observed variable value (not the predicted mean) to compute the distance function. Also, the well-known method of nearest neighbor imputation is similar to PMN, except the distance function is in terms of the original predictor variables and often requires somewhat arbitrary scaling of discrete variables. PMN is a combination of a model-assisted imputation methodology and a random nearest neighbor hot-deck procedure. The hot-deck procedure within the PMN method ensures missing values are imputed to be consistent with nonmissing values for other variables. Whenever feasible, the imputation of variables using PMN is multivariate, in which imputation is accomplished on several response variables at once.
For most variables starting a new baseline for trends in 2015, a modified version of PMN was adopted and continued to be used for these variables in 2020. This procedure also was adopted for all SUD variables beginning in 2020.26 In addition, the questionnaire since 2015 has included questions about any use of prescription drugs in the past year and lifetime periods (i.e., not just misuse of prescription drugs). Consequently, imputation-revised variables have been created since 2015 using this modified version of PMN for any use of prescription pain relievers, tranquilizers, stimulants, and sedatives. Levels in these new variables indicate any past year use, lifetime but not past year use, and lifetime nonuse. Because of changes in how respondents are asked about the initiation of misuse of prescription drugs, imputation-revised variables for the age at first misuse and the date of first misuse have been created since 2015 only for past year initiates. For nonprescription drugs and for prescription drugs prior to 2015, age at first use (or misuse) and the date of first use (or misuse) were created for all lifetime users of the drug of interest.
While still utilizing the model-assisted imputation methodology described previously, modified PMN involves collocated stochastic imputation (CSI)27 for categorical variables based on the predicted probabilities from the modeling step. Under modified PMN, nonspecific or missing values for continuous variables are still assigned using a donor selected from a hot-deck procedure. One benefit of modified PMN is the ability to cycle through a group of variables being imputed as a set. This cycling process allows variables imputed later in the sequence to be used as covariates in the modeling process for variables earlier in the sequence, thus reducing the importance of imputation order.
Variables imputed using PMN for 2020 were (1) the initial demographic variables; (2) substance use variables for cigarettes, smokeless tobacco, cigars, pipe tobacco, alcohol, marijuana, cocaine, crack, and heroin (recency of use, frequency of use, and age at first use); (3) income; (4) health insurance; and (5) demographic variables for work status, immigrant status, and the household roster. Variables imputed using modified PMN for 2020 were the drug use variables for hallucinogens, inhalants, methamphetamine, pain relievers, tranquilizers, stimulants, and sedatives (recency of any use, recency of misuse, frequency of misuse, past year initiation status, and age at first misuse among past year initiates of misuse). Additionally, modified PMN was used in 2020 to impute variables related to DSM-5 SUD outcomes28 (i.e., past year disorder and disorder severity) for alcohol and illicit drugs and the most recent use of the following: vaping of any substance, nicotine or tobacco vaping, kratom, synthetic marijuana, and synthetic stimulants.
In the modeling stage, the model chosen depends on the nature of the response variable. In the 2020 NSDUH, the models included binomial logistic regression, multinomial logistic regression, Poisson regression, time-to-event (survival) regression, and ordinary linear regression, where the models incorporated the sampling design weights.
In general, hot-deck imputation replaces an item nonresponse (missing or nonspecific value) with a recorded response donated from a “similar” respondent who has nonmissing data. For random nearest neighbor hot-deck imputation, the missing or nonspecific value is replaced by a value from a donor respondent who was randomly selected from a set of potential donors. Potential donors are those defined to be “close” to the unit with the missing or nonspecific value according to a predefined function called a distance metric. In the hot-deck procedure of PMN or modified PMN for continuous variables, the set of candidate donors (the “neighborhood”) consists of respondents with complete data who have a predicted mean close to that of the item nonrespondent. The predicted means are computed both for respondents with and without missing data, which differs from Rubin’s method where predicted means are not computed for the donor respondent (Rubin, 1986). In particular, the neighborhood consists of either the set of the closest 30 respondents or the set of respondents with a predicted mean (or means) within 5 percent of the predicted mean(s) of the item nonrespondent, whichever set is smaller. If no respondents are available who have a predicted mean (or means) within 5 percent of the item nonrespondent, the respondent with the predicted mean(s) closest to that of the item nonrespondent is selected as the donor.
In the univariate hot-deck situation (where only one variable is imputed), the neighborhood of potential donors is determined by calculating the relative distance between the predicted mean for an item nonrespondent and the predicted mean for each potential donor, then choosing those means defined by the distance metric. The pool of donors is restricted further to satisfy logical constraints whenever necessary (e.g., age at first crack use must not be less than age at first cocaine use).
Whenever possible, missing or nonspecific values for more than one response variable are considered together when using hot-deck imputation to select a donor. In this (multivariate) situation, the distance metric is a Mahalanobis distance, which takes into account the correlation and heterogeneous variances between variables (Manly, 1986), rather than a Euclidean distance. The Euclidean distance is the square root of the sum of squared differences between each element of the predictive mean vector for the respondent and the predictive mean vector for the nonrespondent. The Mahalanobis distance standardizes the Euclidean distance by the variance-covariance matrix, which is appropriate for correlated random variables or those having heterogeneous variances. Whether the imputation is univariate or multivariate, only missing or nonspecific values are replaced, and donors are restricted to be logically consistent with the response variables that are not missing. Furthermore, donors are restricted to satisfy “likeness constraints” whenever possible. That is, donors are required to have the same values for variables highly correlated with the responses. For example, donors for the age-at-first-use variable are required to be of the same age as recipients, if at all possible. If no donors are available who meet these conditions, these likeness constraints can be loosened. Further details on the PMN methodology are provided by Singh et al. (2002).
Although statistical imputation could not proceed separately within each state due to insufficient pools of donors, information about each respondent’s state of residence was incorporated in the modeling and hot-deck steps. For most drugs, respondents were separated into three “state usage” categories as follows: respondents from states with high usage of a given drug were placed in one category, respondents from states with medium usage into another category, and the remainder into a third category. This categorical “state rank” variable was used as one set of covariates in the imputation models. In addition, eligible donors for each item nonrespondent were restricted to be of the same state usage category (i.e., the same “state rank”) as the nonrespondent.
Under modified PMN, values for categorical variables are assigned using CSI, which selects values randomly based on the predicted means from the prediction model rather than by hot-deck imputation. To ensure consistency across multiple measures, conditional probabilities are used if the imputed value must be restricted. In expectation, under CSI, the weighted mean of the imputed values across all item nonrespondents will be equal to the weighted mean of the predicted means across all item nonrespondents. Utilizing CSI rather than a purely independent random selection reduces the probability of unusual results by ensuring the random numbers are spread out evenly between 0 and 1 and helps to preserve the distribution.
Before 2020, approximately 90 percent of variables undergoing statistical imputation typically required less than 5 percent of their records to be logically assigned or statistically imputed. For the 2020 NSDUH, most variables undergoing statistical imputation (approximately 75 percent of imputed variables) required less than 5 percent of their records to be logically assigned or statistically imputed. Variables for measures highly sensitive or perhaps not known to younger respondents (e.g., family income) often have higher rates of item nonresponse. In addition, certain variables subject to a greater number of skip patterns and consistency checks (e.g., frequency of use in the past 12 months and past 30 days) often require greater amounts of imputation.
2.3.3.2 Special Imputation Issues for the 2020 NSDUH
For the 2020 NSDUH, the imputation procedures were revised to account for the methodological changes in response to the COVID-19 pandemic that were discussed in Sections 2.1 and 2.2. These modifications sought to address possible differences in response patterns between (1) respondents interviewed in person and those who completed the survey via the web and (2) respondents interviewed during Quarter 1 and those who completed the survey during Quarter 4. To incorporate information about data collection quarter and interview mode into the imputation procedures, the following changes were made for 2020:
- The imputation procedures were implemented separately for the Quarter 1 and Quarter 4 data.
- For the Quarter 4 imputations, the data collection mode (i.e., in person or web) was included as a covariate in the response propensity and prediction models for PMN and modified PMN imputations.
- Tables 2.3 and 2.4 at the end of this chapter summarize the distributions of the weighted statistical imputation rates of these variables by interview section in Quarters 1 and 4, respectively.
- As noted previously, modified PMN also was used to impute missing data for DSM-5 SUD variables for alcohol and all illicit drugs. This change was made because SUD estimates using DSM-5 criteria started a new baseline for 2020.
Other than these changes, the imputation methods that were implemented in 2020 were equivalent to those used in 2019 and earlier years. However, with the smaller sample size in 2020, imputation models often included fewer covariates. Higher rates of item nonresponse for certain variables also led to higher imputation rates, as shown in Tables 2.3 and 2.4.
2.3.4 Development of Analysis Weights
This section discusses the approach used to develop NSDUH person-level analysis weights29 for 2020. Changes to the 2020 data collection procedures that were described previously (e.g., very limited data collection from late March to September and the introduction of multiple modes of data collection beginning in October 2020) affected the weighting procedures for the 2020 NSDUH. Section 2.3.4.1 provides an overview of the general weighting procedures. Details about specific changes to the 2020 weighting process are discussed in Section 2.3.4.2.
2.3.4.1 General Weighting Approach
The general approach to developing NSDUH person-level analysis weights involves two types of components: design-based weights and weight adjustment factors. Design-based weights were created for the 2020 NSDUH to reflect probabilities of selection at the five sample stages described in Section 2.1.1 and Figure 2.1. The weight adjustment factors calibrate the design-based weights, 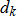 , to reduce bias due to nonresponse, to poststratify to known population control totals, and to control for extreme weights when necessary.
, to reduce bias due to nonresponse, to poststratify to known population control totals, and to control for extreme weights when necessary.
Weight adjustments were based on a generalization of Deville and Särndal’s (1992) logit model. This generalized exponential model (GEM) (Folsom & Singh, 2000) incorporates unit-specific bounds, 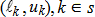 , for the adjustment factor
, for the adjustment factor 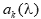 as follows:
as follows:
where 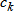 are prespecified centering constants, such that
are prespecified centering constants, such that 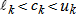 and
and 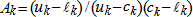 . The variables
. The variables 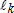 ,
, 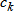 , and
, and 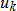 are unit-specific bounds, and
are unit-specific bounds, and 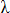 is the column vector of model parameters corresponding to the covariates in vector x. The
is the column vector of model parameters corresponding to the covariates in vector x. The 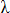 parameters are estimated by solving
parameters are estimated by solving
where 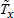 denotes control totals that could be either nonrandom, as is generally the case with poststratification, or random, as is generally the case for nonresponse adjustment.
denotes control totals that could be either nonrandom, as is generally the case with poststratification, or random, as is generally the case for nonresponse adjustment.
The adjustment factor 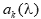 is derived by minimizing the distance function,
is derived by minimizing the distance function,  , defined as
, defined as
The adjusted weights are the product of unadjusted weights (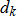 ) and the adjustment factor,
) and the adjustment factor, 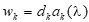 . This general approach was used at several stages of the weight adjustment process, including (1) adjustment of household weights for nonresponse at the screener level, (2) poststratification of household weights to meet population controls for various household-level demographics by state,30 (3) adjustment of household weights for extremes, (4) poststratification of selected person weights,31 (5) adjustment of responding person weights for nonresponse at the questionnaire level, (6) poststratification of responding person weights to census population estimates, and (7) adjustment of responding person weights for extremes.
. This general approach was used at several stages of the weight adjustment process, including (1) adjustment of household weights for nonresponse at the screener level, (2) poststratification of household weights to meet population controls for various household-level demographics by state,30 (3) adjustment of household weights for extremes, (4) poststratification of selected person weights,31 (5) adjustment of responding person weights for nonresponse at the questionnaire level, (6) poststratification of responding person weights to census population estimates, and (7) adjustment of responding person weights for extremes.
Every effort was made to include as many relevant state-specific covariates (typically defined by demographic domains within states) as possible in the multivariate models used to calibrate the weights (nonresponse adjustment and poststratification steps). Because further subdivision of state samples by demographic covariates often produced small cell sample sizes, it was not possible to retain all state-specific covariates (even after meaningful collapsing of covariate categories) and still estimate the necessary model parameters with reasonable precision. Therefore, a hierarchical structure was used in grouping states with covariates defined at the national level, at the census division level within the nation, at the state group within the census division, and, whenever possible, at the state level. In every case, the controls for the total population within a state and the five age groups (12 to 17, 18 to 25, 26 to 34, 35 to 49, 50 or older) within a state were maintained, except in the last step of poststratification of person weights in which six age groups (12 to 17, 18 to 25, 26 to 34, 35 to 49, 50 to 64, 65 or older) were used. Census control totals by age, race, gender, and Hispanic origin were required for the civilian, noninstitutionalized population of each state. The Population Estimates Branch of the U.S. Census Bureau has produced the necessary population estimates for the same year as each NSDUH in response to a special request. Census control totals for the 2020 NSDUH weights were based on population estimates from the 2010 decennial census because control totals based on the 2020 decennial census were not yet available.
GEM has a built-in extreme weight control algorithm that applies tighter bounds to the predetermined extreme weights in the nonresponse and poststratification adjustments (CBHSQ, 2021c). This method is unlike the traditional method of winsorization in which extreme weights are truncated at prespecified levels and the trimmed portions of weights are distributed to the nontruncated cases. In GEM, bounds can be set around the prespecified levels for extreme weights. Then the calibration process provides an objective way of deciding the extent of adjustment (or truncation) within the specified bounds.
2.3.4.2 Special Weighting Issues for the 2020 NSDUH
The general methodology and procedures described in Section 2.3.4.1 were applied to develop the person-level analysis weights for the 2020 NSDUH. In addition to these general procedures, the weighting process for the 2020 NSDUH included modifications to account for the disruption in data collection due to the COVID-19 pandemic and the subsequent introduction of multimode data collection (in person and web) for NSDUH in Quarter 4.
The first modification was to add an implicit unknown eligibility adjustment if SDUs selected for web-based data collection were nonrespondents at the screening stage. When data are collected in person, FIs can identify ineligible SDUs (e.g., vacancies) to allow the ineligible SDUs to be excluded from the sample before weighting. For web-based data collection, however, SDU members needed to initiate the screening process. Consequently, web-based data collection yielded more SDUs with unknown eligibility because an adult SDU member did not contact RTI to begin the screening process. If an SDU’s eligibility was unknown for web-based data collection, its eligibility status was imputed according to the historic SDU eligibility rate in the state where the SDU was located. SDUs that were imputed to be ineligible were excluded from the weighting process before the SDU nonresponse adjustment.
The second modification was that separate analysis weights were developed for Quarter 1 (January to March) and for Quarter 4 (October to December) of 2020. Separate Quarter 1 and Quarter 4 analysis weights were created because (1) new questionnaire items were added in Quarter 4, and (2) nonresponse patterns were different between Quarter 1 and Quarter 4 due to different data collection modes in Quarter 4. Separate weights needed to be developed for Quarter 4 to generate estimates from data that were available only in Quarter 4 (see Section 2.2.2.3). The analysis weights for combined Quarter 1 and Quarter 4 data were created by combining the two quarters of data and dividing the nonzero Quarter 1 and Quarter 4 analysis weights by a factor of 2. For measures that were available from both quarters of 2020, published estimates in NSDUH tables and reports typically used the analysis weights for the combined Quarter 1 and Quarter 4 data, unless noted otherwise.
The third modification for the 2020 person-level weights was the addition of educational attainment to the poststratification adjustment models for 2020. In Quarter 4, the web data for educational attainment (less than high school, high school graduate, some college or associate degree, and college graduate) showed a higher percentage of college graduates and a somewhat smaller proportion of adults with a high school education or less compared with distributions from prior NSDUH years and distributions from the American Community Survey (ACS). The educational distribution for in-person data from Quarter 1 and prior NSDUH years aligned well with ACS distributions. Therefore, the 2019 ACS data were used to create control totals for educational attainment. The control totals for educational attainment were obtained by multiplying the ACS educational attainment proportions by the 2020 civilian, noninstitutionalized population estimates received from the U.S. Census Bureau. Educational attainment was added to the poststratification adjustment models for Quarter 1 and Quarter 4.
A fourth modification was needed for the 2020 person-level weights to account for the number of adult web respondents in Quarter 4 who provided usable information on their substance use (see Section 2.3.1) but did not complete the full interview (i.e., “break-offs”). In the 2020 NSDUH, 8.6 percent of respondents aged 12 or older in Quarter 4 met the usability criteria described in Section 2.3.1, but they did not complete the interview. The corresponding percentage in Quarter 1 was 0.05 percent. Further investigation of the Quarter 4 data indicated that adults rather than adolescents tended to break off the interview. The higher percentage of respondents (principally adults) in Quarter 4 who broke off the interview (which was driven by web-based data) suggested it would not be correct to assume that missing data for adults occurred completely at random starting with the adult mental health section of the questionnaire.
The mental health outcome variables were not statistically imputed, but some measures underwent zero imputation, where missing values were assumed to be equivalent to negative responses (see Section 3.3.2.2). If the number of missing values is small, performing zero imputation for relatively uncommon outcomes introduces negative bias but has very little effect (CBHSQ, 2017b). However, with mental health and later data from Quarter 4 having an increased percentage of respondents with missing data because of interview break-offs, these missing data could contribute to a meaningful bias in estimates. Therefore, additional break-off analysis weights were created to analyze the unimputed outcomes starting from the mental health and subsequent sections of the questionnaire. Table 2.5 shows a list of questionnaire sections starting with the mental health section and whether any variables in that section were imputed.
Respondents were classified into two groups: (1) break-off respondents and (2) non-break-off respondents. Break-off respondents did not complete the adult depression section of the interview (for adults aged 18 or older) or the youth experiences section of the interview (for adolescents aged 12 to 17). Non-break-off respondents completed the interview or broke off the interview after the adult depression section (for adults) or the youth experiences section (for adolescents).
Break-off analysis weights were developed for Quarter 1 and Quarter 4 separately. The break-off analysis weights were created for Quarter 4 using data from the non-break-off adult respondents by performing an additional poststratification adjustment using GEM on the main analysis weights. The Quarter 4 main analysis weights of the non-break-off adult respondents were poststratified to the Quarter 4 main analysis weights for all adult respondents. The break-off analysis weights for break-off adult respondents were set to zero, and their weights were redistributed among the weights for the non-break-off adult respondents during the poststratification adjustment. In addition to the same demographic totals used in the previous poststratification adjustment models, three outcome variables were added to the control totals for the additional break-off poststratification adjustment: past month alcohol use, past month cigarette use, and any lifetime pain reliever use (i.e., use or misuse).
As noted previously, only 0.05 percent of Quarter 1 respondents met the usability criteria but did not complete the interview. To simplify the process for Quarter 1, therefore, the break-off analysis weights for Quarter 1 were developed using the weighting class adjustment method. The main analysis weights for the non-break-off adult respondents were ratio adjusted to the main analysis weights of all adult respondents within state by age group domains.
In both Quarter 1 and Quarter 4, the break-off poststratification adjustment was not performed for interview data from youths aged 12 to 17 because few youths broke off the interview. Therefore, the 2020 break-off analysis weights were the same as the main analysis weights for youths.
Break-off analysis weights were also created for combined data from Quarters 1 and 4. These weights were calculated by combining the two quarters of data and dividing nonzero break-off analysis weights from Quarter 1 and Quarter 4 by a factor of 2.
Figure 2.1 Stratification and Stages of Selection
 D
D
DC = District of Columbia; SSR = state sampling region.
3. Statistical Methods and Measurement
3.1 Target Population
The estimates of the prevalence of substance use and mental health issues from the National Survey on Drug Use and Health (NSDUH) are designed to describe the target population of the survey—the civilian, noninstitutionalized population aged 12 years or older living in the United States. This population covers residents of households (people living in houses or townhouses, apartments, condominiums; civilians living in housing on military bases; etc.) and people in noninstitutional group quarters (e.g., shelters, rooming or boarding houses, college dormitories, migratory workers’ camps, halfway houses). In particular, the 2010 census reported there were 308.7 million people of all ages living in the United States in 2010, of whom 300.8 million were living in households, or about 97 percent of the total population of the United States (Lofquist et al., 2012). Thus, the civilian, noninstitutionalized population aged 12 years or older would be expected to include at least 97 percent of the total U.S. population aged 12 years or older.
However, the civilian, noninstitutionalized population excludes some small subpopulations that may have very different estimates of mental disorders and substance use and therefore may have specific issues or needs. For example, the survey excludes active military personnel, who may be exposed to combat situations or stressors associated with extended overseas deployment. In addition, military personnel have been shown to have significantly lower rates of illicit drug use but higher rates of heavy alcohol use compared with their counterparts in the civilian population (Bray et al., 2009). The survey also excludes people living in institutional group quarters, such as jails or prisons, residential substance abuse treatment or mental health facilities, nursing homes, and long-term hospitals. People in some of these institutional settings may have higher rates of mental disorders or substance use disorders (SUDs) compared with the general population. Another subpopulation excluded from NSDUH consists of people with no fixed address (e.g., homeless and/or transient people not living in shelters); homeless people are another population shown to have higher than average rates of mental disorders and substance use problems (Bassuk et al., 2015; Solari et al., 2014). Chapter 5 of this report describes other surveys providing substance use and mental health data for these populations.
3.2 Estimation and Statistical Significance
The sampling error of an estimate is the error caused by the selection of a sample instead of conducting a census of the population. The sampling error may be reduced by selecting a large sample, by using efficient sample design and estimation strategies (such as stratification, optimal allocation, and ratio estimation), or by taking both approaches. The use of probability sampling methods in NSDUH allows estimation of sampling error from the survey data.
Estimates based on NSDUH data are presented in reports and in sets of tables referred to as “detailed tables” available at https://www.samhsa.gov/data/. The national estimates, along with the associated standard errors (SEs), which are the square roots of the variances, were computed for all detailed tables using a multiprocedure package, SUDAAN® Software for Statistical Analysis of Correlated Data. This software uses a Taylor series linearization approach to account for the effects of NSDUH’s complex sample design features in estimating the SEs (RTI International, 2013). The SEs are used to identify unreliable estimates and to test for the statistical significance of differences between estimates. The final, nonresponse-adjusted, and poststratified analysis weights were used in SUDAAN to compute unbiased, design-based estimates. See Section 2.3.4 for more information on the 2020 analysis weights.
3.2.1 Variance Estimation for Estimated Numbers of People
The variances and SEs of estimates of means and proportions can be calculated reasonably well in SUDAAN using a Taylor series linearization approach. Estimates of means or proportions, , such as drug use prevalence estimates for a domain d, can be expressed as a ratio estimate,
, such as drug use prevalence estimates for a domain d, can be expressed as a ratio estimate,
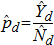 , D
, D
where 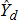 is a linear statistic estimating the number of people with the characteristic of interest (e.g., substance users) in the domain d and
is a linear statistic estimating the number of people with the characteristic of interest (e.g., substance users) in the domain d and 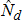 is a linear statistic estimating the total number of people in domain d (including people with or without the characteristic of interest, such as substance users and nonusers). The SUDAAN software package is used to calculate direct estimates of
is a linear statistic estimating the total number of people in domain d (including people with or without the characteristic of interest, such as substance users and nonusers). The SUDAAN software package is used to calculate direct estimates of 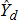 and
and 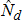 (and, therefore,
(and, therefore, 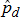 ) and also can be used to estimate their respective SEs. A Taylor series approximation method implemented in SUDAAN provides the estimate for the SE of
) and also can be used to estimate their respective SEs. A Taylor series approximation method implemented in SUDAAN provides the estimate for the SE of 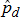 .
.
When the domain size,  , is assumed to be free of sampling error because the domain size has been fixed (see below), the following formula is an alternative to using SUDAAN to estimate the SE for the total number of people with a characteristic of interest (e.g., substance users):
, is assumed to be free of sampling error because the domain size has been fixed (see below), the following formula is an alternative to using SUDAAN to estimate the SE for the total number of people with a characteristic of interest (e.g., substance users):
This alternative SE estimation method is theoretically correct when the domain size estimates, 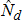 , are fixed (i.e., among those domains forced to match their respective U.S. Census Bureau or American Community Survey population estimates through the weight calibration process). In these situations,
, are fixed (i.e., among those domains forced to match their respective U.S. Census Bureau or American Community Survey population estimates through the weight calibration process). In these situations, 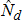 is not subject to a sampling error induced by the NSDUH design. That is, the U.S. Census Bureau and American Community Survey population estimates are assumed to be free of sampling error induced by the NSDUH design. Sections 2.3.4 and 6.2.2 in this report contain further information about the weight calibration process. In addition, more detailed information about the weighting procedures for 2020 will appear in the 2020 NSDUH methodological resource book (Center for Behavioral Health Statistics and Quality [CBHSQ], forthcoming a). Until that volume becomes available, refer to the 2019 NSDUH methodological resource book (CBHSQ, 2021a) for general weighting information; however, specific information about the 2020 weighting procedures will not be available in the 2019 volume.
is not subject to a sampling error induced by the NSDUH design. That is, the U.S. Census Bureau and American Community Survey population estimates are assumed to be free of sampling error induced by the NSDUH design. Sections 2.3.4 and 6.2.2 in this report contain further information about the weight calibration process. In addition, more detailed information about the weighting procedures for 2020 will appear in the 2020 NSDUH methodological resource book (Center for Behavioral Health Statistics and Quality [CBHSQ], forthcoming a). Until that volume becomes available, refer to the 2019 NSDUH methodological resource book (CBHSQ, 2021a) for general weighting information; however, specific information about the 2020 weighting procedures will not be available in the 2019 volume.
For an estimated number 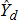 where the domain
where the domain 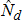 is nonfixed (i.e., where domain size estimates are not forced to match the U.S. Census Bureau or American Community Survey population estimates), this alternative SE estimation method still may provide a good approximation if it can be assumed the sampling variation in
is nonfixed (i.e., where domain size estimates are not forced to match the U.S. Census Bureau or American Community Survey population estimates), this alternative SE estimation method still may provide a good approximation if it can be assumed the sampling variation in 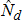 is negligible relative to the sampling variation in
is negligible relative to the sampling variation in 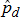 . This is a reasonable assumption for many situations in this study.
. This is a reasonable assumption for many situations in this study.
For some subsets of domain estimates, using this alternative SE estimation method where domain sizes are nonfixed yielded an underestimate of the SE of the total when 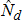 was subject to considerable variation. Because of this underestimation, the alternative SE estimation method was not implemented when
was subject to considerable variation. Because of this underestimation, the alternative SE estimation method was not implemented when  was nonfixed.
was nonfixed.
Since the 2005 NSDUH, a “mixed-method” approach has been implemented for all detailed tables to improve the accuracy of SEs and to better reflect the effects of poststratification on the variance of the total estimated numbers of people. This approach assigns the methods of SE calculation to domains (i.e., subgroups for which the estimates were calculated) within tables so that all estimates among a select set of domains with fixed 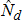 were calculated using the alternative SE estimation method, and all other estimates were calculated directly in SUDAAN, regardless of what the other estimates are within the same table. The set of domains with a fixed
were calculated using the alternative SE estimation method, and all other estimates were calculated directly in SUDAAN, regardless of what the other estimates are within the same table. The set of domains with a fixed 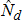 was restricted to main effects and two-way interactions to maintain continuity between years.32 The use of such SEs for the estimated numbers of people did not affect the SE estimates for the corresponding proportions presented in the same sets of tables because all SEs for means and proportions are calculated directly in SUDAAN.
was restricted to main effects and two-way interactions to maintain continuity between years.32 The use of such SEs for the estimated numbers of people did not affect the SE estimates for the corresponding proportions presented in the same sets of tables because all SEs for means and proportions are calculated directly in SUDAAN.
Table 3.1 at the end of this chapter includes the domains that employed the alternative SE estimation method, including the main domains and the two-way interactions. Table 3.1 presents main domains and two-way interactions for the combined data from Quarters 1 and 4 and separately for Quarter 1 and Quarter 4.33 However, Table 3.1 does not include an exhaustive list of domains and interactions for which estimates are presented in NSDUH reports and detailed tables. For domains not included in Table 3.1, SEs for the estimates of totals are calculated directly in SUDAAN. For example, Tables 8.2 and 8.5 in the 2020 detailed tables present estimates of any mental illness (AMI) and serious mental illness (SMI), respectively, among adults aged 18 or older within the domains of gender, Hispanic origin and race, and current employment. Estimated numbers of adults with AMI or SMI among the total population and age group (age group is the main effect), males and females (age group by gender interaction), and Hispanics and non-Hispanics (age group by Hispanic origin interaction) used the alternative SE estimation method to calculate the SEs. The SEs for all other estimated numbers of people in Tables 8.2 and 8.5 in the 2020 detailed tables, including current employment, were calculated directly in SUDAAN. Similarly, SEs by age group for Whites (three-way interactions of age by Hispanic origin by race interaction) were calculated directly in SUDAAN. It is important to note that estimates presented in the detailed tables for racial groups are among non-Hispanics, unless noted otherwise. For instance, the domain for Whites is actually non-Hispanic Whites and is therefore a two-way interaction.
3.2.2 Suppression Criteria for Unreliable Estimates
As has been done in past survey years, direct estimates from NSDUH designated as unreliable are not shown in reports or tables and are noted by asterisks (*). The criteria used to define unreliability of direct estimates from NSDUH are based on the prevalence (for proportion estimates), relative standard error (RSE) (defined as the ratio of the SE over the estimate), nominal (actual) sample size, and effective sample size for each estimate. These suppression criteria for various NSDUH estimates are summarized in Table 3.2 at the end of this chapter.34
Proportion estimates ( ), or rates, within the range [
), or rates, within the range [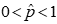 ], and the corresponding estimated numbers of users were suppressed if
], and the corresponding estimated numbers of users were suppressed if
The threshold of .175 in the above rule was chosen because it equates with a suppression threshold based on an effective sample size of 68 when  = .05, .50, or .95 (i.e., if the threshold were increased, then that would equate with a lower suppression threshold based on an effective sample size, and vice versa).
= .05, .50, or .95 (i.e., if the threshold were increased, then that would equate with a lower suppression threshold based on an effective sample size, and vice versa).
Using a first-order Taylor series approximation to estimate 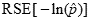 and
and 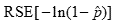 , the following equation was derived and used for computational purposes when applying a suppression rule dependent on effective sample size:35
, the following equation was derived and used for computational purposes when applying a suppression rule dependent on effective sample size:35
The separate formulas for 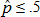 and
and 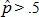 produce a symmetric suppression rule; that is, if
produce a symmetric suppression rule; that is, if  is suppressed,
is suppressed, 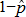 will be suppressed as well (see Figure 3.1 following Table 3.2).36 Figure 3.1 also illustrates how this suppression rule can equivalently be expressed as a suppression rule based on the effective sample size as a function of
will be suppressed as well (see Figure 3.1 following Table 3.2).36 Figure 3.1 also illustrates how this suppression rule can equivalently be expressed as a suppression rule based on the effective sample size as a function of  . The figure shows that when
. The figure shows that when 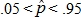 , the symmetric properties of the rule produce a local minimum effective sample size of 50 at
, the symmetric properties of the rule produce a local minimum effective sample size of 50 at  = .2 and at
= .2 and at  = .8; however, as
= .8; however, as  moves away from these two points, then the suppression threshold increases to a maximum of an effective sample size of 68 reached at
moves away from these two points, then the suppression threshold increases to a maximum of an effective sample size of 68 reached at  = .05 or .95, or at the local maximum,
= .05 or .95, or at the local maximum,  = .50. Therefore, to simplify requirements and maintain a conservative suppression rule, estimates of
= .50. Therefore, to simplify requirements and maintain a conservative suppression rule, estimates of  = .05 between .05 and .95 were suppressed if they had an effective sample size below 68 (indicated by a horizontal line at 68 in Figure 3.1); the suppression rule was left unchanged for estimates of
= .05 between .05 and .95 were suppressed if they had an effective sample size below 68 (indicated by a horizontal line at 68 in Figure 3.1); the suppression rule was left unchanged for estimates of  outside of this range, which will require increasingly larger effective sample sizes in order to avoid suppression. For example, an effective sample size of 153, 232, and 684 is needed when
outside of this range, which will require increasingly larger effective sample sizes in order to avoid suppression. For example, an effective sample size of 153, 232, and 684 is needed when  = .01, .005, and .001, respectively.
= .01, .005, and .001, respectively.
In addition, a minimum nominal sample size suppression criterion (n = 100) was employed to protect against unreliable estimates caused by small design effects and small nominal sample sizes; Table 3.2 shows a formula for calculating design effects. Prevalence estimates also were suppressed if they were close to 0 or 100 percent (i.e., if  < .00005 or if
< .00005 or if  > .99995).
> .99995).
The suppression rule for proportions based on 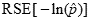 described previously replaced a rule in which data were suppressed whenever
described previously replaced a rule in which data were suppressed whenever 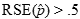 . This rule was changed because the previous RSE rule imposed a very stringent application for suppressing estimates when
. This rule was changed because the previous RSE rule imposed a very stringent application for suppressing estimates when  is small but imposed a very lax application for large
is small but imposed a very lax application for large  . The replacement rule ensured a more uniformly stringent application across the whole range of
. The replacement rule ensured a more uniformly stringent application across the whole range of  (i.e., from 0 to 1). The previous rule also was asymmetric in the sense that suppression only occurred in terms of
(i.e., from 0 to 1). The previous rule also was asymmetric in the sense that suppression only occurred in terms of  . That is, there was no complementary rule for (
. That is, there was no complementary rule for (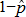 ), which the current NSDUH suppression criteria for proportions take into account.
), which the current NSDUH suppression criteria for proportions take into account.
Estimates of totals were suppressed if the corresponding prevalence rates were suppressed. Given this rule, data users may encounter some unexpected results after applying the suppression rules. One such result may occur when equivalent estimates of totals corresponding to different estimated percentages,  , are suppressed differently. To demonstrate, consider a table presenting estimates of past month substance use among the population aged 12 or older and different age groups (e.g., 12 to 17, 18 to 25, 26 or older), where
, are suppressed differently. To demonstrate, consider a table presenting estimates of past month substance use among the population aged 12 or older and different age groups (e.g., 12 to 17, 18 to 25, 26 or older), where  for the population aged 12 or older is not suppressed, but
for the population aged 12 or older is not suppressed, but  for the 12 to 17 age group is suppressed. Thus, the estimated total would be displayed for the 12 or older age group and would be suppressed for the 12 to 17 age group. However, if
for the 12 to 17 age group is suppressed. Thus, the estimated total would be displayed for the 12 or older age group and would be suppressed for the 12 to 17 age group. However, if  were suppressed for the total population and the 12 to 17 age group, then both of the estimated totals would be suppressed as well. Another unexpected result may occur when
were suppressed for the total population and the 12 to 17 age group, then both of the estimated totals would be suppressed as well. Another unexpected result may occur when  is not suppressed, but the estimated total is displayed as a zero (0). Because the estimated totals are shown as numbers in thousands, a zero actually represents an estimated number greater than zero but less than 500, which is appropriately displayed because
is not suppressed, but the estimated total is displayed as a zero (0). Because the estimated totals are shown as numbers in thousands, a zero actually represents an estimated number greater than zero but less than 500, which is appropriately displayed because  was not suppressed.
was not suppressed.
Estimates of means not bounded between 0 and 1 (e.g., mean of age at first use, mean number of days of use in the past 30 days or past 12 months) were suppressed if the RSEs of the estimates were larger than .5 or if the nominal sample size was smaller than 10 respondents. This rule was based on an empirical examination of the estimates of mean age of first use and their SEs for various empirical sample sizes. Although arbitrary, a sample size of 10 appeared to provide sufficient precision and still allow reporting by age at first use for many substances.
3.2.3 Statistical Significance of Differences
This section describes the methods used to compare prevalence estimates. Customarily, the observed difference between estimates is evaluated in terms of its statistical significance. Statistical significance is based on the p value of the test statistic and refers to the probability that a difference as large as that observed would occur due to random variability in the estimates if there were no differences in the prevalence estimates being compared. The significance of observed differences is typically reported at the .05 level. When comparing prevalence estimates, the null hypothesis (no difference between prevalence estimates) was tested against the alternative hypothesis (there is a difference in prevalence estimates) using the standard t test (with the appropriate degrees of freedom) for the difference in proportions test, expressed as
where df = the appropriate degrees of freedom, 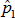 = the first prevalence estimate,
= the first prevalence estimate, 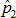 = the second prevalence estimate,
= the second prevalence estimate, 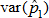 = the estimated variance of the first prevalence estimate,
= the estimated variance of the first prevalence estimate, 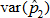 = the estimated variance of the second prevalence estimate, and
= the estimated variance of the second prevalence estimate, and 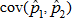 = the estimated covariance between
= the estimated covariance between 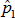 and
and 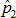 . This formula applies to significance tests between subgroups within a single year (e.g., between males and females in 2020) and tests between estimates in different survey years.37 In situations where significance tests between years were performed, the prevalence estimate from the earlier year becomes the first prevalence estimate, and the prevalence estimate from the later year becomes the second prevalence estimate (e.g., 2018 is the first estimate, and 2019 is the second).
. This formula applies to significance tests between subgroups within a single year (e.g., between males and females in 2020) and tests between estimates in different survey years.37 In situations where significance tests between years were performed, the prevalence estimate from the earlier year becomes the first prevalence estimate, and the prevalence estimate from the later year becomes the second prevalence estimate (e.g., 2018 is the first estimate, and 2019 is the second).
Under the null hypothesis, the test statistic t is a random variable asymptotically following a t distribution. Therefore, calculated values of t, along with the appropriate degrees of freedom, can be used to determine the corresponding probability level (i.e., p value). Whether testing for differences between years or from different populations within the same year, the covariance term in the formula for t will, in general, not be equal to zero. SUDAAN was used to compute estimates of t along with the associated p values using the analysis weights and accounting for the sample design as described in Chapter 2 of this report. A similar procedure and a similar formula for t were used for estimated numbers of people with a characteristic of interest.
Under the null hypothesis, the test statistic with known variances asymptotically follows a standard normal (Z) distribution. However, because the variances of the test statistic are estimated, its distribution is more accurately described by the t distribution for finite sample sizes. As the degrees of freedom approach infinity, the t distribution approaches the Z distribution. Because most tests performed for the 2020 NSDUH have 750 degrees of freedom,38 the t tests performed produce approximately the same numerical results as if a Z test had been performed (CBHSQ, 2021d).
Caution is needed when reviewing multiple years of data for estimated numbers of people with a characteristic of interest. Respondents with large analysis weights can greatly influence the estimated number in a given year when the number of people in the population with that characteristic is relatively small (e.g., past month heroin users). Large analysis weights for some respondents in a single year can result in the estimated numbers of people with a given characteristic showing an increase between Year 1 and Year 2 (i.e., the year that had the respondents with large analysis weights) but then decreasing in Year 3 back to an estimated number that is similar to that in Year 1.39 The potential for these kinds of year-to-year variations in estimated numbers of people also underscores the importance of reviewing changes across a larger range of years, especially for outcome measures corresponding to a relatively small proportion of the total population.
A second caution is needed when reviewing multiple years of data for estimated numbers of people. A change in the estimated number of people with a characteristic of interest could reflect a change in the size of the overall population. Therefore, changes in estimated numbers of people should be considered in conjunction with the corresponding estimated percentages because percentages will control for changes in both the number of people with the characteristic of interest and the total number of people in the population. If corresponding percentages are not available (e.g., for estimates of the number of past year initiates), caution should be taken in interpreting changes over time, which may be explained by population increases rather than by true increases in the characteristic of interest.
For the 2020 NSDUH, statistical tests were conducted among population subgroups within a single year. When comparing population subgroups across three or more levels of a categorical variable (e.g., age group, race/ethnicity), log-linear chi-square tests of independence of the subgroups and the prevalence variables were conducted using SUDAAN in order to first control the error level for multiple comparisons. If, and only if, Shah’s Wald F test (transformed from the standard Wald chi-square) indicated overall significant differences, the significance of each particular pairwise comparison of interest was tested using SUDAAN analytic procedures to properly account for the sample design (RTI International, 2013). This two-step procedure protected against inappropriate inferences being drawn due to the number of pairwise differences tested.40 Although these tests are generally not published in the detailed tables, they may be used for NSDUH reports to document statistically significant differences across subgroups (e.g., by age group).
Using the published estimates and SEs to perform independent t tests for the difference of proportions will typically provide similar results as tests performed in SUDAAN. However, results may differ for two reasons: (1) the covariance term is included in SUDAAN tests, whereas it is not included in independent t tests; and (2) the reduced number of significant digits shown in the published estimates may cause rounding errors in the independent t tests.
Significance testing also was conducted using SUDAAN to compare estimates for individual subgroups with the corresponding estimate among the overall population (e.g., northeast region vs. all regions). In the 2020 detailed tables, these significance tests were conducted for select demographic measures (i.e., race/Hispanic origin and region). However, comparing estimates between a subgroup and the overall population increases the covariance in the denominator of the t test formula described at the beginning of this section; subtracting this covariance term from the sum of the variance terms for the individual estimates will decrease the size of the denominator and increase the size of the t statistic. For this reason, small differences between a subgroup and the overall population can be statistically significant. These tests could be used to aid authors in writing NSDUH reports, but they are not published in the detailed tables.
3.3 Other Information on Data Accuracy
The accuracy of survey estimates can be affected by nonresponse, coding errors, computer processing errors, errors in the sampling frame, reporting errors, and other errors not due to sampling. They are sometimes referred to as “nonsampling errors.” These types of errors and their impact are reduced through data editing, statistical adjustments for nonresponse, close monitoring and periodic retraining of interviewers, and improvement in various quality control procedures.
Although these types of errors often can be much larger than sampling errors, measurement of most of these errors is difficult. However, some indication of the effects of some types of these errors can be obtained through proxy measures, such as response rates, and from other research studies. For effects of different modes of data collection within a survey (Section 3.3.3), analyses can compare data across modes.
3.3.1 Screening and Interview Response Rate Patterns
All sampled households are screened to confirm eligibility and to select zero, one, or two household members to participate in the survey. The weighted screening response rate (SRR) is defined as the weighted number of successfully screened households41 divided by the weighted number of eligible households (as defined in Table 3.3), or
where 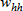 is the inverse of the unconditional probability of selection for the household and excludes all adjustments for nonresponse and poststratification defined in Section 2.3.4 of this report.
is the inverse of the unconditional probability of selection for the household and excludes all adjustments for nonresponse and poststratification defined in Section 2.3.4 of this report.
For the 2020 NSDUH, household eligibility was imputed for households whose members did not initiate the web screening interview and that were not visited by a field interviewer (FI) (i.e., households with unknown eligibility). Thus, the weighted SRR is equivalent to the response rate 4 (RR4) in the American Association for Public Opinion Research (AAPOR) standard definitions (AAPOR, 2016), which estimates the proportion of sampled households with unknown eligibility that are actually eligible. That is,
where I is the weighted sum of the successfully screened households, P is the weighted sum of the partially screened households, R is the weighted sum of the refusals and break-offs, NC is the weighted sum of the noncontacts, O is the weighted sum of the other eligible nonresponding households, e is the estimated proportion of sampled households with unknown eligibility that are eligible (estimated using historical NSDUH data), UH is the weighted sum of the sampled addresses in which it is unknown if an eligible housing unit exists, and UO is the weighted sum of the households in which it is unknown if an eligible person is present in the housing unit. According to the definition of a successfully screened household, no partially screened households are in NSDUH’s SRRs (i.e., the letter P in AAPOR’s RR4). Thus, RR4 becomes 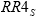 , or
, or
In successfully screened households, eligible household members who were selected were then asked to complete the interview. The weighted interview response rate (IRR) for NSDUH is defined as the weighted number of respondents divided by the weighted number of selected people (see Tables 3.4 and 3.5), or
where 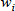 is the inverse of the probability of selection for the person and includes household-level nonresponse and poststratification adjustments (adjustments 1, 2, and 3 in Section 2.3.4.1). In an effort to maximize IRRs, all respondents were offered a $30 incentive to encourage them to complete the 2020 NSDUH interview. To be considered a completed interview, a respondent for the 2020 NSDUH needed to provide enough data to pass the usability criteria described in Section 2.3.1.
is the inverse of the probability of selection for the person and includes household-level nonresponse and poststratification adjustments (adjustments 1, 2, and 3 in Section 2.3.4.1). In an effort to maximize IRRs, all respondents were offered a $30 incentive to encourage them to complete the 2020 NSDUH interview. To be considered a completed interview, a respondent for the 2020 NSDUH needed to provide enough data to pass the usability criteria described in Section 2.3.1.
Similar to the weighted SRR, the weighted IRR is equivalent to the AAPOR standard definition RR2, except that all of the respondents have known eligibility. Thus, the weighted IRR can be written as 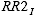 , which is based on the AAPOR definition, or
, which is based on the AAPOR definition, or
where I is the weighted sum of the completed interviews, P is the weighted sum of the partial interviews (with enough data to pass the usable case rule), R is the weighted sum of the refusals and break-offs failing the usable case rule, NC is the weighted sum of the noncontacts, and O is the weighted sum of the other eligible nonrespondents.
The overall weighted response rate (ORR) is defined as the product of the weighted SRR and weighted IRR, or
3.3.1.1 2020 NSDUH Response Rates
In the first calendar quarter of the 2020 NSDUH (January to March), data were collected using standard in-person methods as in prior years (Section 2.2.1.1.1). As discussed in Chapter 2, in-person data collection was suspended on March 16, 2020, because of the coronavirus disease 2019 (COVID-19) pandemic. Quarter 4 data were collected using multimode (web and in-person) data collection (see Sections 2.1.2.2, 2.2.1.1.2, and 2.2.1.3). Thus, the two data collection periods had different response patterns.
Of the 52,827 eligible sampled households in Quarter 1, 35,304 were screened successfully, for a weighted SRR of 67.8 percent (Table 3.3). In these screened households, 24,304 people were selected, and 15,628 completed the NSDUH interview. The Quarter 1 weighted IRR and ORR were 63.2, and 42.9 percent, respectively (Table 3.5).
Although the addition of web-based data collection in Quarter 4 increased the number of interviews that were completed, web-based data collection yielded lower response rates than in-person data collection. Further, Quarter 4 in-person response rates were negatively affected by the COVID-19 pandemic. Specifically, the pandemic could have exacerbated people’s reluctance to open their doors to FIs in areas where in-person interviewing was allowed. FIs also could have had a shorter amount of time to follow up with households that started out as being eligible for in-person data collection but became ineligible based on changing state- and county-level health metrics.
In Quarter 4, screening was completed with 55,633 of the 483,376 eligible sampled households, for a weighted SRR of 11.1 percent (Table 3.3). A total of 38,211 people were selected for the NSDUH interview, and 20,656 people completed the interview. The weighted IRR and ORR were 59.5 and 6.6 percent, respectively (Table 3.6).
For the full 2020 sample from both quarters, a total of 536,203 sampled households were eligible. Of these eligible sampled households, 90,937 were screened successfully, for a weighted SRR of 25.7 percent (Table 3.3). In the 90,937 screened households, a total of 62,515 people were selected, and completed interviews were obtained from 36,284 of these sampled people, for a weighted IRR of 60.4 percent and a weighted ORR of 15.5 percent (see Table 3.4). A total of 7,746 sampled people (10.2 percent) were classified as refusals or parental refusals; 16,719 (25.7 percent) were not available, never at home, or did not respond to the web survey (Quarter 4 only); and 1,766 (3.6 percent) did not participate for various other reasons, such as partially completed but unusable interviews (Section 2.3.1), physical or mental incapacity, or language barrier (see Table 3.4). Tables 3.5 and 3.6 show the distribution of the selected sample by interview code and age group. Among demographic subgroups, the weighted IRR was higher among people aged 26 or older (63.7 percent), females (63.1 percent), Whites (62.6 percent), people in the Midwest (62.6 percent), and residents of nonmetropolitan areas (62.3 percent) than among their corresponding counterparts (Table 3.7).
3.3.1.2 Bias Due to Unit Nonresponse
Maximizing NSDUH response rates is intended to minimize biases in estimates due to different characteristics of respondents and nonrespondents. Drug use surveys may be particularly vulnerable to nonresponse bias if recent or frequent drug users are less likely to participate in the survey, especially for less commonly used substances such as crack cocaine or heroin. However, a study that matched 1990 census data to 1990 National Household Survey on Drug Abuse (NHSDA) nonrespondents42 found that populations with low response rates did not always have high drug use rates (Gfroerer et al., 1997a). For example, although some populations were found to have low response rates and high drug use rates (e.g., residents of large metropolitan areas and males), other populations had low response rates and low drug use rates (e.g., older adults and high-income populations). These earlier findings suggest that potential sources of nonresponse bias in one direction (e.g., bias that would decrease estimates) could be offset by corresponding sources of bias in the opposite direction (e.g., bias that would increase estimates), such that overall effects on prevalence because of nonresponse bias could be minimal. Further research on this important topic with recent NSDUH data would be useful.
As discussed in Sections 2.3.4.2 and 3.3.1.1, web-based data collection in Quarter 4 for the 2020 NSDUH resulted in lower response rates and yielded respondents with higher educational attainment compared with results from in-person data collection. To address potential nonresponse bias from sample members with less education being less likely to participate via the web, education was included in the poststratification adjustments for weighting the 2020 data (see Sections 2.3.4.2 and 6.2.2.2).
3.3.2 Item Nonresponse and Inconsistent Responses
3.3.2.1 Item Nonresponse
Among survey participants, item response rates were generally very high for most mental health and drug use items. With the introduction of web-based data collection in Quarter 4 of 2020, however, item nonresponse rates increased due to respondents discontinuing the survey prior to completion (i.e., breaking off). See Section 2.3.4.2 for details.
People who started the survey but broke off the interview before completing a minimum number of drug use questions were not kept as final respondents based on the usability criteria described in Section 2.3.1. Therefore, item nonresponse tended to be lower for drug use items and higher for mental health items. Prior to Quarter 4 of 2020, item nonresponse was predominantly caused by responses of “don’t know” or “refused” either in the specific question or in an earlier question that governed skip logic.43 In Quarter 4 of 2020, item nonresponse among adults was driven by survey break-offs.
For example, 0.4 percent of the adult respondents interviewed in person in 2020 (regardless of quarter) had missing data (e.g., no response, responses of “don’t know” or “refused”) for whether they received mental health services in the past 12 months as an inpatient. In comparison, 4.1 percent of adults in Quarter 4 who completed the survey via the web had missing data for the same measure, for an overall rate of 2.5 percent for this item for all adult respondents in 2020. Among adults in 2020 with missing data for whether they received inpatient mental health services, 10 percent had missing data because of break-offs for in-person respondents compared with 99 percent of web respondents.
Similarly, 2.7 percent of adult respondents in 2020 had missing data for whether they received outpatient mental health services in the past 12 months (0.6 percent for in-person respondents and 4.3 percent of web respondents). Also, about 3.5 percent of adults had missing data for the question about serious thoughts of suicide in the past 12 months (about 0.6 percent for in-person respondents and about 5.5 percent for web respondents).
About 3.9 to 4.3 percent of adults in the full 2020 sample had missing data for questions about specific lifetime symptoms of depression; the highest percentages for missing data (4.3 and 4.2 percent) among the depression items occurred in the questions about the specific number of pounds respondents lost without trying to lose weight (question AD26f in the adult depression section) and whether anyone noticed respondents were talking or moving slowly (question AD26m), respectively.44 Among adult in-person respondents the range of missing data for lifetime symptoms of depression ranged from 1.0 to 1.5 percent. The range for adult web respondents in Quarter 4 was 6.0 to 6.4 percent.
Break-offs contributed less to item nonresponse for respondents aged 12 to 17 than for adults in the 2020 NSDUH. Unlike the adult data, item nonresponse was lower for youths who completed the survey via the web instrument than it was for those who were interviewed in person.
Among youths in the full 2020 sample, about 1.0 to 1.7 percent had missing data for whether they received mental health services in the past 12 months from specific sources other than through schools (1.1 to 1.9 percent for in-person respondents and 0.7 to 1.2 percent for web respondents); about 1.8 percent had missing data for the receipt of school-based services (2.2 percent for in-person respondents and 0.8 to 0.9 percent for web respondents). About 2.5 to 3.9 percent had missing data for questions about specific lifetime symptoms of depression (2.0 to 4.1 percent for in-person respondents and 2.0 to 3.5 for web respondents); the highest percentage of youths in the full sample who had missing data for the depression items (3.9 percent) occurred in the question about the specific number of pounds youths lost without trying to lose weight (4.1 percent among in-person respondents and 3.5 percent among web respondents).
Among the full sample of respondents aged 12 or older in 2020, 2.5 percent had missing data for whether they received treatment for use of alcohol or illicit drugs in their lifetime and in the past 12 months (1.7 percent of in-person respondents and 3.2 percent of web respondents). About 1.5 percent of respondents had missing data for whether they ever received substance use treatment because they broke off the interview prior to the drug treatment questions, and 0.9 percent had missing data because their status as a lifetime user of alcohol or illicit drugs was unknown.
Patterns of missing data for the lifetime receipt of substance use treatment differed somewhat for in-person and web respondents. Among in-person respondents in the full sample, 0.02 percent had missing data for whether they ever received substance use treatment because they broke off the interview, and 1.4 percent had missing data because their status as lifetime substance users was unknown. Among web respondents, 2.7 percent had missing data because they broke off the interview, and 0.4 percent had missing data because of their status for lifetime alcohol or illicit drug use was unknown.
In addition, item nonresponse becomes important for measures created from multiple questions because nonresponse to a single item can result in the overall measure being assigned a missing value. For example, respondents aged 12 to 17 who reported receiving mental health services in either of two inpatient mental health settings (any type of hospital or a residential treatment center) were asked to report the number of nights they stayed in a given facility in the past 12 months. An overall measure of the number of nights spent in either setting in the past 12 months would have a missing value if there was insufficient information across both items to determine the total number of nights spent in either of these settings. In the 2020 NSDUH, 17.1 percent of respondents aged 12 to 17 who reported receiving treatment at an inpatient facility had missing data for the total number of nights they spent in any inpatient facility for mental health care in the past 12 months (19.1 percent among in-person respondents in the full sample and 9.7 percent among web respondents in Quarter 4).
Responses of “don’t know” also may suggest an underlying characteristic of respondents. In questions such as the perceived risk of harm from the use of different substances or the perceived availability of substances, responses of “don’t know” may be a valid response category for respondents. Respondents may not have formed an opinion on the topic, or they may have no knowledge of the substance.
In 2020, about 0.6 percent of respondents aged 12 or older and about 1.4 to 1.6 percent of adolescent respondents aged 12 to 17 answered “don’t know” to questions about the perceived risk of harm from smoking a pack or more of cigarettes a day, having four or five drinks of an alcoholic beverage nearly every day, or having five or more drinks of an alcoholic beverage once or twice a week.45 For the perceived risk of harm from using different illicit drugs, percentages of respondents aged 12 or older in the full 2020 sample who answered “don’t know” ranged from 1.0 percent for the perceived risk from using marijuana (once a month or once or twice a week) to 2.1 percent for the perceived risk from trying lysergic acid diethylamide (LSD) or using LSD once or twice a week. Among adolescent respondents aged 12 to 17, the percentage who answered “don’t know” ranged from 1.9 percent for the perceived risk from using marijuana once or twice a week to 5.3 percent for the perceived risk from trying LSD.
Moreover, in 2020, responses of “don’t know” to questions about the perceived risk of harm from substance use continued to be the predominant source of missing data in the majority of these questions. Among respondents aged 12 or older who were asked the perceived risk questions and had missing data (i.e., excluding respondents who broke off prior to or during this questionnaire section), 87 to 89 percent answered “don’t know” to the questions regarding the perceived risk of harm from cigarette and alcohol use, and 92 to 95 percent answered “don’t know” to the questions regarding the risk of perceived harm from using different illicit drugs. Responses of “don’t know” to questions about the perceived risk of harm from using these substances could reflect a general lack of knowledge about these substances.
Among respondents aged 12 or older in 2020 who were asked the perceived availability questions for illicit drugs and had missing data, 94 percent did not know how easy or difficult it would be to get illicit drugs other than marijuana. Among respondents who were asked about the perceived availability of marijuana and had missing data, 88 percent did not know how easy or difficult it would be to get marijuana. Not knowing how difficult or easy it would be to obtain these substances could indicate a predisposition not to use them.
Aside from issues of potential biases discussed below, excluding respondents with missing data for perceived risk and availability measures (especially those who answered “don’t know”) could have other implications for published estimates. For example, excluding respondents who answered “don’t know” to these questions might create the impression that all people in the population have an opinion about the perceived risk of harm from substance use or the perceived availability of different substances. For these measures, the percentage of people who did not know how to answer these questions could be useful information.
3.3.2.2 Effects of Missing Data on Estimates
If statistical imputation were not used to replace missing values with nonmissing values (see Section 2.3.3), then the variables serving as the starting point for creating NSDUH estimates would have some missing data. Generally, observations with missing values are excluded from standard NSDUH analyses, including a portion (but not all) of the analyses used to create the annual detailed tables.
For some variables, however, missing values are assumed to be equivalent to negative responses, such as assuming respondents with missing data for a given symptom of psychological distress in the past 30 days or past 12 months did not have that symptom (see Section 3.4.7). The issue is exacerbated when a measure is constructed from multiple variables in which missing values are assumed equivalent to negative responses, such as for AMI and SMI.46 The assumption that missing data are equivalent to negative responses causes a negative bias. The magnitude of the bias depends on both the percentage of respondents with missing data and the magnitude of the estimate. Specifically, a high level of nonresponse and a high estimate induce a large negative bias. A low level of nonresponse and a low estimate induce a small negative bias. Intermediate combinations induce a moderate negative bias.
Several variables for which missing data are treated as being equivalent to a negative response are described in Section 3.4. To mitigate the effect in Quarter 4 of increased rates of item nonresponse due to break-offs when missing data are assumed to be equivalent to negative responses, a break-off analysis weight was created to use with unimputed data for adults who broke off the interview at or before the adult depression section (see Sections 2.3.4.2 and 6.2.2.3).
Bias also may result when respondents with missing data are excluded from the analysis. This issue is discussed in more detail in the 2019 NSDUH statistical inference report (CBHSQ, 2021d).
For estimated numbers of people with a given characteristic, a negative bias will always occur if there are missing values in the domain variables, the outcome variable, or both. For example, estimates of exposure of youths aged 12 to 17 to school-based substance use prevention messages include a domain variable consisting of youths who attended school in the past 12 months (including those who were home schooled), and the outcome variables, which consist of whether youths received substance use prevention messages in various school settings. Both the domain and the outcome variables may have missing data, and respondents with missing data for school attendance or exposure to school-based prevention activities were excluded from the analyses.
When a population mean or a population proportion is estimated, there may or may not be bias, and the bias can be negative or positive. The direction and magnitude of the bias for means and proportions depend on how different the item respondents are from the item nonrespondents with respect to the outcome of interest. For example, if “true” perceptions of the risk of harm from the use of different substances (i.e., no risk, slight risk, moderate risk, great risk) among respondents with missing data matched the distribution of respondents who did not have missing data, then excluding missing data (and decreasing the number of respondents in the denominator) would be expected to increase the estimated percentage of people in the population who perceived great risk of harm from using a substance. However, if the actual perceived risk of harm among respondents with missing data was skewed in favor of perceived great risk of harm, for example, then excluding these missing data might introduce other biases in published estimates.
3.3.2.3 Inconsistent Responses
In order to minimize respondent confusion, inconsistent responses, and item nonresponse, the NSDUH computer-assisted interviewing (CAI) instrument is programmed to skip respondents out of inapplicable questions based on their previous answers. This skip logic reduced the potential for inconsistent data by limiting respondents’ opportunity to provide answers that were inconsistent with previous answers. For example, if adult respondents did not report staying overnight in a hospital or other facility to receive mental health services in the past 12 months, they were not asked questions about the type of inpatient facility where they received mental health services, the number of nights they spent in inpatient facilities, or the payment sources for their inpatient mental health services in that period. Thus, respondents could not report they did not receive inpatient mental health services in the past 12 months and then answer one or more of these additional questions as though they had.
Similarly, questions added to the 2020 NSDUH for when respondents last vaped nicotine or tobacco were tailored according to respondents’ reports of when they last vaped any substance. This tailoring of questions was designed to reduce the opportunity for respondents to provide answers for when they last vaped nicotine or tobacco that were inconsistent with their reports of when they last vaped any substance (see Section 3.4.12.2).
However, programming of skip patterns within the CAI instrument did not eliminate all occurrences of missing or inconsistent data. For example, when asked about cocaine use, respondents who reported not knowing whether they had ever used cocaine are not asked further questions about this substance, resulting in missing data for their most recent use and when they initiated use. Respondents also could report lifetime use of cocaine but give inconclusive information (i.e., responses of “don’t know” or “refused”) for when they last used it. Consequently, information is unknown for whether these lifetime users used cocaine in the past year or past month. Similarly, respondents could give inconsistent responses, such as reporting they last used any form of cocaine more than 12 months ago but they last used crack cocaine in the past 30 days or last used it more than 30 days ago but within the past 12 months; both answers logically cannot be true.
These missing or inconsistent responses in the substance use data are first resolved where possible through a logical editing process (e.g., logically inferring more recent reported use of crack cocaine applies to any cocaine). Additionally, missing or inconsistent responses for substance use are imputed using statistical methodology. These imputation procedures in NSDUH are based on responses to multiple questions, so that other relevant information is utilized through statistical modeling when determining whether a respondent is classified as a user or nonuser—and, if the respondent is classified as a user, whether the respondent is classified as having used a substance in the past year or the past month. For example, nonspecific data on the most recent use of cocaine are statistically imputed based on a respondent’s data for use (or most recent use) of tobacco products, alcohol, and marijuana. Nevertheless, editing and imputation of missing responses are potential sources of measurement error.
As for the substance use variables, the CAI skip logic also did not eliminate all opportunities for inconsistent reports in the mental health questions. Consequently, the logical editing procedures for the mental health data could slightly increase the amount of missing data when inconsistent answers were given. For example, if adult or adolescent respondents who met the criteria for a lifetime major depressive episode (MDE) (see Section 3.4.8) reported an age at onset for depression symptoms47 greater than their current age, the inconsistent age-at-onset variable was set to a missing value. However, the number of respondents in 2020 with this inconsistency was small (i.e., fewer than 10 respondents aged 12 or older).
In addition, not all inconsistencies in the data are resolved through editing or imputation. Inconsistencies could remain when questions are asked in different questionnaire sections. For example, respondents could indicate in the COVID-19 section added in Quarter 4 of 2020 that they experienced specific situations in accessing substance use treatment because of the COVID-19 pandemic, but they previously did not report lifetime use of alcohol or illicit drugs. In situations such as these, data users would need to decide how to handle these inconsistencies in their analyses.
For more information on editing and statistical imputation, see Sections 2.3.2 and 2.3.3 in this report. Details of the editing and imputation procedures for 2020 also will appear in the 2020 NSDUH methodological resource book (CBHSQ, forthcoming a). Until that volume becomes available, refer to the 2019 NSDUH methodological resource book (CBHSQ, 2021a) for the most recent documentation of general principles and procedures for editing and imputation.
3.3.3 Mode Effects
The 2020 NSDUH for the first time included self-administered interviews collected via the web, in addition to the standard in-person data collection from prior years (Section 2.2.1). The web mode was introduced in Quarter 4 out of necessity because in-person data collection posed unreasonable health risks for respondents and FIs48 in most geographical areas during the COVID-19 pandemic. Although the in-person and web questionnaires had similar content as much as possible, Section 2.2.2.4 describes notable differences for in-person and self-administered web interviews.
To recap briefly, if sample dwelling units (SDUs) were selected for web-based data collection during Quarter 4,49 FIs were not involved in (1) contacting SDUs, (2) obtaining consent for an eligible adult to screen the SDU, (3) ensuring that data collection transitioned correctly from the screening respondent to the individuals who were selected for the main interview, or (4) obtaining consent for the main interview (including parental permission if the selected individual was aged 12 to 17). FIs also were not available during web-based data collection to answer questions on the spot from respondents. Although web-based interviews were self-administered (as were most sections for in-person interviews), respondents who completed the web interviews did not have the benefit of being able to listen to audio recordings of self-administered questions. Members of SDUs also needed Internet access to complete the survey via the web. Consequently, as discussed in Section 2.3.4.2, adults in Quarter 4 who completed the interview via the web were more likely to be college graduates compared with adults in Quarter 4 who completed the interview in person.
Such differences between data collection modes can lead to “mode effects,” or differences in respondent characteristics and response patterns between the in-person and web modes. For the 2020 NSDUH, however, potential effects of the COVID-19 pandemic on substance use and mental health outcomes were completely confounded with these mode effects. When important methodological changes are contemplated for a survey, including a controlled experiment can test the effects of the changes. For the 2020 NSDUH, however, a controlled experiment was not feasible because of the suspension of in-person data collection starting in March 2020 due to COVID-19. When data collection resumed in October 2020 (except for a very small number of interviews that were conducted in July 2020), it would not have been ethical to randomly assign SDUs for in-person or web-based data collection in locations where COVID-19 infection rates made in-person data collection unsafe.
Analyses described in the remainder of this section were conducted to attempt to tease out the independent effects of the data collection modes and events in the population in 2020, including the pandemic and other social upheavals. These analyses included examining overall effects due to the mode change and specific comparisons between in-person and web modes using data from Quarter 4 of 2020.
3.3.3.1 Overall Effects of the Mode Change
Overall screening and interview response rates were lower in 2020 during the pandemic than in previous years, regardless of mode. For example, the weighted SRR in Quarter 1 (before in-person data collection was suspended in mid-March 2020) among people aged 12 or older was 67.8 percent, the weighted IRR was 63.2 percent, and the ORR was 42.9 percent (Section 3.3.1.1). In 2019, weighted response rates across all four quarters among people aged 12 or older were 70.5 percent for the SRR, 64.9 percent for the IRR, and 45.8 percent for the ORR (CBHSQ, 2020c).
Among adults in 2020, the IRR in Quarter 4 (62.9 percent) was nearly the same as in Quarter 1 (62.5 percent) (Tables 3.5 and 3.6), despite most data collection in Quarter 4 having been web-based. Among youths aged 12 to 17, the IRR was lower in Quarter 4 than in Quarter 1 (25.6 vs. 70.5 percent). The lower IRR in Quarter 4 among youths was likely because of the steps needed for obtaining parental permission and assent from adolescent respondents before web-based data collection could proceed (see Section 2.2.1.3).
Also, the SRR in Quarter 4 was substantially lower than in Quarter 1 (11.1 vs. 67.8 percent) (Table 3.3). The lower SRR in Quarter 4 is likely explained by SDU members being responsible for starting the screening process for web-based data collection, whereas FIs contacted SDUs for in-person data collection. Consequently, the ORR in Quarter 4 for the population aged 12 or older was only 6.6 percent compared with the rate of 45.8 percent in Quarter 1. However, lower response rates are not atypical for web-based data collection compared with in-person data collection. For example, response rates among adults in the experimental 2020 Household Pulse Survey ranged from 1.3 to 3.8 percent in the first 3 weeks of data collection (see Section 5.1.3) (National Center for Health Statistics, n.d.-a). The web-based 2015 Department of Defense (DoD) Health Related Behaviors Survey had an overall response rate of 8.6 percent (Meadows et al., 2018).
Demographic characteristics of web and in-person respondents were similar, with one important exception. As noted previously, adults in Quarter 4 who completed the interview via the web had higher levels of education compared with in-person respondents. Therefore, education level was added to the control totals for poststratification of person-level analysis weights to bring the distribution of education levels back into alignment with the population (Section 2.3.4.2).
NSDUH web respondents in Quarter 4 also were more likely to “break off” the interview without completing it; break-offs are common in web surveys (e.g., Lozar-Manfreda & Vehovar, 2002; Mavletova & Couper, 2015; Peytchev, 2009). If a respondent opened the web questionnaire and immediately decided not to complete it, the interview failed to pass the usability criteria. Consequently, the web mode had more unusable interviews. However, if the respondent got far enough into the questionnaire before breaking off, the interview would be deemed usable but would still have missing values from the break-off point forward. Thus, the web interviews had more missing data than in-person interviews had. Missing values due to breaking off also greatly exceeded missing values from responses of “don’t know” or “refused.” Due to the large number of break-offs in the web data, one concern was that missing data due to respondents breaking off were not missing completely at random, but instead were related to the nature of the questions, particularly the mental health questions. Therefore, an additional set of analysis weights was developed for estimating mental illness and MDE among adults and for estimates from later measures from the interview that did not undergo statistical imputation (see Section 2.3.4.2).50
3.3.3.2 Quarter 4 Mode Effects
Analyses were conducted using preliminary Quarter 4 data from 2020 and preliminary analysis weights for a set of 20 key substance use and mental health outcomes.51 Estimates for seven out of the 20 key outcomes, including three with a past month reference period, were significantly different from those estimates of the corresponding 2020 Quarter 1 data. Similarly, the estimates for the same three past month outcomes for Quarter 4 2020 were significantly different from the corresponding 2019 Quarter 4 estimates. See Section 6.3.1 and Table 6.8. These differences could be caused by the addition of the web mode in 2020, influences of the COVID-19 pandemic and other social factors, or seasonality in the data. Analysis of quarterly estimates of the same outcomes from prior years indicated some significant changes across quarters but showed that very few of the significant differences were consistent across years. That is, some seasonality is possible, but seasonal differences were too inconsistent to conclude that the observed differences in Quarter 4 of 2020 were explained by seasonality. A comparison of estimates from in-person interviews in 2020 Quarter 4 with estimates from the same segments in 2019 (all in person) also showed very little difference in estimates. However, the locations in which in-person data collection occurred in Quarter 4 of 2020 had acceptably low COVID-19 infection rates. The pandemic may not have had much impact in those particular areas, but one cannot generally conclude that the pandemic had no impact.
A limited set of statistical models was run to compare odds ratios and risk ratios for multiple quarters of preliminary data for the same 20 key binary outcomes noted previously. The models indicated a tendency for web respondents to be less likely to report substance use (across a combination of age groups), but respondents in Quarter 4 of 2020 were more likely to report substance use (i.e., during a period of increased COVID-19 infection rates). That is, effects due to the mode change and effects due to COVID-19 may have worked in opposite directions on some of the outcomes. Although these results cannot be considered definitive, two independent modeling exercises hinted at similar tendencies. As noted previously, however, these modeling investigations were not extensive and were conducted using preliminary data and preliminary analysis weights. Therefore, results from these initial investigations may not generalize to all 2020 NSDUH data and to all population subgroups. Also, odds ratios and risk ratios from these investigations indicate the general direction of influences of the data collection mode change and elapsed time during the pandemic for the outcomes that were investigated, but they cannot be used to construe what the 2020 estimates would have been if all data had been collected in person.
In summary, data users should exercise caution when comparing the 2020 NSDUH estimates with estimates from prior years because the reasons for differences in estimates across years cannot be determined conclusively. The COVID-19 pandemic and the resulting addition of the web mode of data collection in Quarter 4 happened in tandem without the benefit of a controlled experiment to measure the effects of each. As mentioned elsewhere in the report, data collection in 2020 also differed from that in prior years because of the pause in data collection in the middle of 2020. For these reasons, the Substance Abuse and Mental Health Services Administration (SAMHSA) decided not to compare 2020 estimates with those from prior years in the detailed tables and key substance use and mental health indicators report for the 2020 NSDUH (CBHSQ, 2021e, 2021h).
3.3.4 Validity of Self-Reported Substance Use
Most estimates of substance use, including those produced for NSDUH, are based on self-reports of use. This section focuses on the validity of NSDUH respondents’ self-reports of substance use and is not intended to provide a comprehensive discussion of issues associated with the validity of any self-report in NSDUH. Factors such as the length of time between an event and the interview date or respondents’ interpretation of a question also can affect respondents’ recall or reporting, independent of the potential sensitivity of the topic covered by a question. An additional factor discussed in this section is the use of different data collection modes (i.e., in person or web) used in Quarter 4 of 2020 (October to December) because of the COVID-19 pandemic.
Survey questions about topics such as substance use are considered to be sensitive because respondents may think the questions are intrusive (“none of your business”), pose risks for negative social or legal consequences if their answers were to become known, or require them to provide socially undesirable answers (Tourangeau & Yan, 2007). Although studies generally have supported the validity of self-report data for sensitive topics, the potential for these data to be biased (underreported or overreported) is well documented. The bias varies by several factors, including the mode of administration, the setting, perceptions of privacy, the population under investigation, and for substance use, the type of drug (Aquilino, 1994; Brener et al., 2006; CBHSQ, 2012b; Harrison & Hughes, 1997; Lindberg & Scott, 2018; Tourangeau & Smith, 1996; Tourangeau & Yan, 2007; Turner et al., 1992). NSDUH utilizes widely accepted methodological practices for increasing the accuracy of self-reports, such as encouraging privacy through self-administration of questions about sensitive topics, including audio computer-assisted self-interviewing (ACASI) for in-person data collection, and providing assurances that individual responses will remain confidential. Comparisons using these methods within NSDUH data (collected in person) have shown they reduce reporting bias (Gfroerer et al., 2002).
3.3.4.1 Comparison of Self-Reports and Biological Specimens
Various procedures also have been used to validate self-report data, such as biological specimens (e.g., urine, hair, saliva), proxy reports (e.g., family member, peer), and repeated measures (e.g., to identify recanting of previous reports of use) (Fendrich et al., 1999). However, these procedures often are impractical or too costly for routine use in general population epidemiological studies (SRNT Subcommittee on Biochemical Verification, 2002). Challenges in collecting biological specimens are especially relevant for NSDUH because of its large sample size and coverage of all 50 states and the District of Columbia.
A special study cosponsored by SAMHSA and the National Institute on Drug Abuse (NIDA) examined the validity of NSDUH self-report data on drug use among people aged 12 to 25. The study found urine and hair specimens can be collected with a relatively high response rate in a general population survey, and most youths and young adults reported their recent drug use accurately in self-reports (Harrison et al., 2007).52 However, some reporting differences were observed in either direction, with some respondents not reporting use but testing positive, and some respondents reporting use but testing negative. Technical and statistical problems related to the hair tests precluded presenting comparisons of self-reports and hair test results. Small sample sizes for self-reports and positive urine test results for opioids and stimulants precluded drawing conclusions about the validity of self-reports of these drugs. Furthermore, inexactness in the window of detection for drugs in biological specimens and biological factors affecting the window of detection could account for some inconsistency between self-reports and urine test results.
More recent studies have not been conducted to compare self-reported substance use in NSDUH with test results from biological specimens. A search in the PubMed database for published English-language literature in the past 5 years also supports prior concerns about the practicality and cost of collecting biological specimens from respondents in general population surveys. Recent studies have tended to focus on special study populations, such as clinical trial participants (Baker et al., 2018; Rowe et al., 2018), former inmates (van den Berg et al., 2018), special housing populations (Rendon et al., 2017, 2019), sexual minorities (Li et al., 2019), or young adults who used heroin or misused prescription opioids (Palamar et al., 2019).
3.3.4.2 Reporting of Sensitive Behaviors in Web Surveys
Prior studies spanning multiple decades (e.g., Aquilino, 1994; Lindberg & Scott, 2018; Tourangeau & Smith, 1996) have established that respondents are more likely to report sensitive behaviors when questions are self-administered than when they need to report their answers to interviewers. With the introduction of web-based data collection to the 2020 NSDUH in October 2020, a related issue concerns the validity of self-reported substance use in self-administered questions asked via the web. Given the use of the web for interactions that involve the sharing of sensitive data (e.g., Social Security numbers, credit card numbers), however, people who are willing to share these kinds of sensitive data on the web might be expected to report information about their substance use in a web-based survey.
Kreuter and colleagues (2008) assessed social desirability bias in the reporting of potentially sensitive academic information using interviewer administration via computer-assisted telephone interviewing (CATI) and self-administration using interactive voice recognition (IVR) and web administration. Under a randomized experimental design, web administration increased the reporting of socially undesirable academic information such as a cumulative grade point average below 2.5 compared with CATI, with IVR yielding results between those of web administration and CATI. Web respondents also were less likely than CATI respondents to falsely deny socially undesirable outcomes relative to information from external data sources. Although this study focused on academic outcomes rather than substance use, it is consistent with other literature showing that self-administered data collection modes—including web-based data collection—yield increased reports of sensitive behaviors compared with interviewer-administered modes.
In addition, the Monitoring the Future (MTF) study used a split-sample design to test two modes of self-administration in the 2018 and 2019 surveys for its longitudinal follow-up of 12th graders as they progressed into adulthood: (1) the standard paper-and-pencil data collection mode that had been used for approximately 40 years and (2) a “web-push” condition in which respondents were first encouraged to complete the questionnaire on the web and then given the option to complete the paper questionnaire if they did not complete it on the web. The response rate in 2019 among people aged 19 to 30 was higher for the web-push condition than for the paper-and-pencil mode. For the 2019 survey, few substance use estimates were significantly different across data collection modes, but the estimates of lifetime and past year marijuana vaping were higher in the web-push condition for people in this age group than for the paper-and-pencil mode (Schulenberg, et al., 2020). In analyses of 2018 MTF data for adults aged 19 to 30, the estimate of nicotine vaping in the past 30 days was higher in the web-push sample, but this difference became nonsignificant in a logistic regression model that adjusted for sociodemographic characteristics. Analyses of the 2018 MTF data that used attrition weights (accounting for attrition, oversampling of drug users for follow-up, and the complex MTF design) found that the only difference between modes was a higher estimate of cigarette use in the past 30 days in the paper-and-pencil sample (Patrick et al., 2021). Based on the results from the split-sample design in 2018 and 2019, MTF researchers have adopted the web-push design for all young adults aged 19 to 30 beginning with the 2020 survey, with paper-and-pencil questionnaires being provided only on request, and to sample members who did not complete the web survey. These MTF findings suggest a sizable proportion of young adults might find the web mode to be a more private and secure mode than paper-and-pencil for reporting some forms of substance use.
However, one issue for web-based data collection in NSDUH concerns privacy in the household setting. As noted previously, respondents’ perceptions of privacy can influence the reporting of sensitive behaviors (Brener et al., 2006; CBHSQ, 2012b). For the 2020 NSDUH, FIs asked in-person respondents to find a private location to complete the survey. Web respondents were asked to be in a private location within the home and to affirm before starting an interview that they were in a private location. Unlike in-person interviews, however, no privacy ratings were available for web-based interviews to indicate whether interviews remained private throughout the entire interview or if not, the extent of time for which the interview was less than private and who else might have been present. It also is not known whether NSDUH web respondents perceived the web mode to be a more private method for answering sensitive questions compared with in-person data collection in a private setting using ACASI, but with an FI present. Therefore, as web-based interviewing is increasingly used for collecting survey data—including data on sensitive topics such as substance use—methodological research would be useful for establishing the factors that encourage or discourage the reporting of sensitive behaviors via the web.
3.3.4.3 Issues for Self-Reporting of Prescription Drug Misuse
In addition, the emphasis on past year rather than lifetime misuse of specific prescription drugs as part of the partial redesign of the 2015 NSDUH questionnaire appears to have affected the validity of estimates for lifetime misuse of prescription psychotherapeutic drugs (see Section C in the methodological summary and definitions report for the 2015 NSDUH; CBHSQ, 2016). Respondents since 2015 who did not misuse prescription psychotherapeutic drugs in the past 12 months were asked fewer questions than in years prior to 2015 to aid them in recalling whether they misused any prescription psychotherapeutic drug in a given category (e.g., prescription pain relievers) in their lifetime. Respondents since 2015 (including those in the 2020 NSDUH) also did not have cues for recalling misuse more than 12 months ago of prescription drugs no longer available by prescription in the United States in 2019 (e.g., sedatives containing methaqualone, such as those with the brand names Quaalude® or Sopor®). Field test results in 2012 and 2013 for the redesigned prescription drug questions found lower estimates of lifetime misuse of prescription psychotherapeutic drugs based on the redesigned questions compared with estimates based on the NSDUH questionnaire fielded in those years (CBHSQ, 2014b, 2014c). Because lifetime prescription drug misuse estimates would not be expected to show much change from year to year, CBHSQ concluded that the redesigned questionnaire structure resulted in underreporting of lifetime misuse of prescription psychotherapeutic drugs since 2015 compared with years prior to 2015. For this reason, estimates of lifetime misuse of prescription psychotherapeutic drugs are not included in the 2020 detailed tables.
The prescription drug questions since 2015 allowed respondents to report any use or misuse in the past 12 months for specific medications within a given psychotherapeutic category (e.g., the benzodiazepine tranquilizers Xanax®, Xanax® XR, generic alprazolam, and generic extended-release alprazolam). These details were presented to respondents to aid them with recall and recognition. Because respondents could have difficulty knowing or remembering whether they took a generic or brand name drug or what type of formulation they took (i.e., immediate release or extended release), these questions capture data for the use or misuse of prescription drugs containing a given active ingredient but not necessarily for the exact drugs respondents took. For example, respondents could report use or misuse of the brand name tranquilizer Xanax® even if they actually took the generic equivalent (i.e., alprazolam). This issue may be especially relevant for respondents who misused prescription drugs by taking them without a prescription of their own. Analytically, therefore, these self-reports are assumed to be reliable for making estimates of the use or misuse of prescription drugs containing a given active ingredient (e.g., tranquilizers containing alprazolam), even if respondents may have misreported the exact drug they used or misused in the past year. Therefore, 2020 NSDUH estimates for the use or misuse of prescription psychotherapeutic drugs in the past year are reported for overall psychotherapeutic drug categories (e.g., tranquilizers) or for subtypes of related drugs (e.g., benzodiazepine tranquilizers, tranquilizers containing alprazolam), but they are generally not reported for specific individual prescription drugs from the NSDUH questionnaire.53
3.3.5 Revised Estimates for 2006 to 2010
During regular data collection and processing checks for the 2011 NSDUH, data errors were identified. These errors resulted from fraudulent data submitted by FIs and affected the data for Pennsylvania (2006 to 2010) and Maryland (2008 and 2009). Although all fraudulent interviews were removed from the data files, the SDUs associated with the falsified interviews were not removed because they were part of the assigned sample. Instead, at the household screening stage, these SDUs were assigned a final screening code of 39 (“Fraudulent Case”) and were treated as incomplete with unknown eligibility. The screening eligibility status for these fraudulent interviews then was imputed. If fraudulent interviews were imputed to be eligible, then they were treated as unit nonrespondents for weighting purposes; however, these interviews were not treated differently from other unit nonrespondents in the weighting process in 2006 to 2010 (see Section 2.3.4 in this report).
Table B.3 in Appendix B of the 2011 mental health findings report (CBHSQ, 2012c) presents screening results for 2010, the last year affected by these errors. Interviews imputed to be eligible are classified with a final code of 39 (“Fraudulent Case”; see Table 3.3 in this report). The interviews imputed to be ineligible did not contribute to the weights and were reported as “Other, Ineligible” in the affected years. Because falsified screening or interview data was treated either as ineligible or as a unit nonrespondent at the screening level, it did not have any associated interview information (see Tables 3.4 to 3.6). However, some estimates for 2006 to 2010 in the national reports from the 2020 NSDUH, as well as other new reports, may differ from corresponding estimates found in some previous reports. Similarly, some estimates for 2006 to 2010 in the 2020 detailed tables may differ from estimates found in previous tables.
These errors had minimal impact on the national estimates and no effect on direct estimates for the other 48 states and the District of Columbia. In reports where model-based small area estimation techniques are used, estimates for all states may be affected, even though the errors were concentrated in only two states. In reports not using model-based estimates, the only estimates appreciably affected are those for Pennsylvania, Maryland, the mid-Atlantic division, and the Northeast region. Tables and estimates based only on data since 2011 are unaffected by these data errors.
The 2020 national reports do not include region-level, division-level, state-level, or model-based estimates. However, previous national NSDUH reports through the 2013 NSDUH show estimates for the Northeast region or mid-Atlantic division (or both). The 2020 detailed tables include region-level estimates for some measures but do not include trend data by region for 2006 to 2010. Nevertheless, corrected single-year estimates based on 2006 to 2010 data and estimates based on pooled data including any of these years may differ from previously published estimates in NSDUH reports or tables.
Caution is advised when comparing data from older reports with data from more recent reports based on corrected data files. As discussed previously, comparisons of estimates for Pennsylvania, Maryland, the mid-Atlantic division, and the Northeast region are of most concern. Comparisons of national data or data for other states and regions are essentially still valid. CBHSQ within SAMHSA produced a selected set of corrected versions of reports and tables. In particular, CBHSQ released a set of modified detailed tables including revised 2006 to 2010 estimates for the mid-Atlantic division and the Northeast region for certain key measures. CBHSQ does not recommend making comparisons between unrevised 2006 to 2010 estimates and estimates based on data for 2011 and subsequent years for the geographic areas of greatest concern.
3.4 Measurement Issues
Several measurement issues associated with the 2020 NSDUH are discussed in this section. Specifically, these issues include the methods for measuring the use and misuse of prescription drugs, the initiation of substance use or misuse of prescription drugs, SUDs, the need for services for substance use and mental health issues, and the definition of county type. Additionally, this section discusses the effects on mental health measures because of questionnaire changes prior to the partial questionnaire redesign for the 2015 NSDUH; comparability of mental health measures for AMI, SMI, MDE, and the use of mental health services in the past 12 months between 2015 and earlier years was not affected by the partial questionnaire redesign for 2015. Starting with Section 3.4.9, issues are discussed for measures added to the NSDUH questionnaire since 2019.
This section also discusses how missing data were handled analytically to produce the estimates found in the 2020 NSDUH reports and tables. Readers are reminded to refer to Section 3.3.2 for a discussion of potential biases in estimates because of missing data, especially when missing values are assumed to be equivalent to negative responses (e.g., assuming respondents with missing data for a given symptom of psychological distress did not have that symptom [see Section 3.4.7]).
3.4.1 Use and Misuse of Prescription Drugs
The prescription drug questions in the NSDUH CAI instrument underwent a series of changes for the 2015 survey. This section presents highlights of the changes to the prescription drug questions. Details about the changes to the prescription drug questions also are summarized in Chapter 4 of this report, in Section C in the methodological summary and definitions report for the 2015 NSDUH (CBHSQ, 2016), and in a separate report on the use and misuse of prescription drugs for the 2015 NSDUH (Hughes et al., 2016). Because of these changes, new baselines were started in 2015 for the use and misuse of prescription psychotherapeutic drugs and for methamphetamine use.
Prescription drug questions since 2015 have first included a set of “screener” questions that asked respondents to report any use of specific prescription drugs in the past 12 months, regardless of the reason. Respondents were then asked about misuse in the past 12 months for the specific prescription drugs they reported using in that period (see the next paragraph). This change simplified the cognitive task for respondents by decomposing (1) whether they used a specific prescription drug for any reason; and (2) if so, whether they used it in a way constituting misuse.54 Data also have been available starting with the 2015 survey for any use of prescription psychotherapeutic drugs in the past 12 months.
In addition, misuse of prescription psychotherapeutic drugs was redefined in 2015 as use “in any way a doctor did not direct you to use it/them.” Respondents since 2015 have been presented with examples of use in any way not directed by a doctor, including (1) use without a prescription of one’s own; (2) use in greater amounts, more often, or longer than told to take a drug; and (3) use in any other way not directed by a doctor. This definition of misuse since 2015 focuses solely on behaviors constituting misuse, independent of other factors, such as respondents’ motivations for those behaviors. This definition also includes overuse of prescribed medication.
Another important change for the 2015 NSDUH was to collect detailed data about the misuse of specific prescription drugs in the past 12 months instead of in the lifetime period. This change allowed for the removal of questions for prescription drugs with historical relevance for estimating lifetime misuse that were last available by prescription in the United States decades ago (e.g., sedatives containing methaqualone, such as the brand name drugs Quaalude® and Sopor®). Prescription drugs being prescribed more often or recently approved also were added to the 2015 questionnaire. These changes better address the information needs of policymakers in federal and state agencies who are concerned with recent misuse of prescription drugs that were available by prescription in the United States. A further benefit of a 12-month time frame is that this time period is closer to the interview date and facilitates recall, thereby allowing for more accurate estimates.
Finally, questions in 2015 about methamphetamine use were moved from the section for the misuse of prescription stimulants to a separate methamphetamine section on the use of methamphetamine rather than its misuse. This change reflects the illegal manufacture of most methamphetamine used in the United States.
Since 2015, variables for any use of prescription drugs in overall psychotherapeutic categories (e.g., pain relievers) in the lifetime and past year periods underwent statistical imputation to remove missing values (see Section 2.3.3). Variables for the lifetime, past year, and past month misuse of prescription drugs in overall psychotherapeutic categories also have been imputed.55 However, certain variables for any use and misuse of prescription drugs have not been imputed and retained missing values. Section 4.3 discusses these prescription drug measures and how missing data were handled. Also, see Section 3.3.2 for a discussion of the potential bias in estimates depending on how missing data were handled.
For all years since 2015, respondents were not counted as having misused “any other” prescription drug in the past year if the only other drugs they specified were over-the-counter (OTC) drugs.56 Beginning in 2017, respondents also were not counted for estimates in the detailed tables as having misused any other prescription drug if (1) the only other drugs they specified corresponded to prescription drug subtypes in the NSDUH questionnaire for that psychotherapeutic category (e.g., pain relievers containing hydrocodone), or (2) they specified only drugs in these subtypes and OTC drugs. For example, respondents since 2017 who specified Vicodin® and OTC drugs (e.g., acetaminophen) as the only other prescription pain relievers they misused in the past year were counted in estimates for the past year misuse of hydrocodone products but were not counted in estimates for the past year misuse of any other pain reliever.
This procedure could not be applied to estimates for any use in the past year (i.e., not necessarily misuse) of other prescription drugs in a psychotherapeutic category because respondents were not asked to specify the names of other drugs they used. Respondents who reported any past year use of other prescription drugs in a given category were asked whether they misused other prescription drugs in that period and were asked to specify which other drugs they misused. However, respondents could have used other prescription drugs for any reason in the past year in addition to the drugs they specified they had misused in the past year. These respondents legitimately would not have specified they misused these other pain relievers. Therefore, if respondents were not counted as being past year misusers of other prescription drugs according to the criteria described above, also not counting them as being past year users could underestimate the prevalence of any past year use of prescription drugs. Additional details about these procedures are described in the 2017 methodological summary and definitions report (CBHSQ, 2018b).
3.4.2 Initiation of Substance Use or Misuse
In NSDUH, initiation refers to the first use of a particular substance. For prescription psychotherapeutic drugs (pain relievers, tranquilizers, stimulants, and sedatives), initiation refers to the first time misuse ever occurred.57 All of the initiation variables used to create published estimates for the 2020 NSDUH underwent statistical imputation to remove missing values (see Section 2.3.3). Therefore, these variables were not subject to the kinds of potential biases because of missing data described in Section 3.3.2.
In 2020, the survey questionnaire collected year and month of first use for recent initiates (i.e., people who used a particular substance for the first time at their current age or the year before their current age). Month, day, and year of birth also were obtained directly or were imputed for item nonrespondents as part of the data postprocessing. Additionally, the date of the interview was recorded for in-person and web respondents.
Past year initiation for the 2020 NSDUH referred to respondents whose date of first use of a substance (or misuse for psychotherapeutic drugs) was within the 12 months prior to their interview date.58 Past year initiation was determined by self-reported past year use, the age at first use, the year and month of recent new use, and the interview date.
Calculations of estimates of past year initiation did not take into account whether respondents initiated substance use while a resident of the United States. This method of calculation allowed for direct comparability with other standard measures of substance use because the populations of interest for the measures will be the same (i.e., both measures examined all possible respondents and were not restricted to those initiating substance use only in the United States).
One important note for initiation estimates is the relationship between the main categories and subcategories of substances (e.g., hallucinogens would be a main category, and LSD, phencyclidine [PCP], and Ecstasy would be subcategories in relation to hallucinogens). For most measures of substance use from the 2020 NSDUH, any member of a subcategory was by necessity a member of the main category (e.g., if respondents were past month users of Ecstasy, then they were also past month users of any hallucinogen). However, this situation was not true for estimates for the initiation of substance use. For example, an individual can initiate use of any hallucinogen, LSD, PCP, or Ecstasy only once. Respondents who initiated use of any hallucinogen more than 12 months ago by definition were not past year initiates of hallucinogen use, even if they initiated use of LSD, PCP, or Ecstasy in the past year.
A similar issue applied to initiation estimates for the aggregate substance use categories for any illicit drug, any prescription psychotherapeutic drug, tranquilizers or sedatives (i.e., as a combined category), benzodiazepines, and opioids (i.e., heroin or prescription pain relievers). People who first misused prescription stimulants in the past 12 months but who first misused prescription pain relievers more than 12 months prior to the interview date would be past year initiates for the misuse of stimulants. These people would not be past year initiates for the misuse of prescription psychotherapeutic drugs or any illicit drug because they had already misused pain relievers more than 12 months ago. Because of the potential for respondents to underreport lifetime (but not past year) misuse of prescription psychotherapeutic drugs (see the section below for the initiation of misuse of prescription psychotherapeutic drugs), however, lifetime (but not past year) misusers of prescription drugs could be misclassified as past year initiates for any illicit drug or other aggregate substance use categories (e.g., opioids) if they reported past year initiation of another illicit drug (e.g., heroin) but failed to report their lifetime misuse of a prescription psychotherapeutic drug (e.g., pain relievers). Section 4.5.3 discusses additional issues for the measurement of initiation of benzodiazepine misuse.
Additionally, NSDUH cannot identify people at risk for initiation of use for either any tobacco product or nicotine vaping. Aggregate measures for the use of tobacco products include the use of cigarettes, smokeless tobacco, cigars, or pipe tobacco. However, respondents were not asked initiation questions for pipe tobacco or nicotine vaping; therefore, the aggregate risk for initiation of use of either any tobacco product or nicotine vaping cannot be determined. For these reasons, the 2020 detailed tables do not show initiation estimates for any illicit drug, any prescription psychotherapeutic drug, opioids, benzodiazepines, the aggregate category for tranquilizers or sedatives, tobacco products, or nicotine vaping.
In addition to estimates of the number of people initiating use of a substance in the past year, 2020 NSDUH estimates were computed for the mean age at first use or misuse among past year initiates of these substances. Unless specified otherwise, estimates of the mean age at initiation in the past 12 months were restricted to people aged 12 to 49 so that these mean age estimates were not influenced by those few respondents who were past year initiates and were aged 50 or older. As a measure of central tendency, means are influenced by the presence of extreme values in the data. Therefore, constraining the mean age estimates to past year initiates aged 12 to 49 was expected to increase the utility of these results to health researchers and analysts by providing a less biased picture of the substance use initiation behaviors among the civilian, noninstitutionalized population in the United States. This constraint was applied only to estimates of mean ages at first use and did not affect estimates for the 2020 NSDUH of the numbers of new users or associated percentages (e.g., the percentage of past year users who initiated use in the past year).
In NSDUH years where trend data are reported,59 caution also is advised in interpreting trends in these mean ages at first use, even if past year initiates aged 26 to 49 were assumed to be less likely than past year initiates aged 50 or older to influence mean ages at first use. Sampling error in initiation estimates for adults aged 26 to 49 can affect year-to-year interpretation of trends (see Section 3.2). Consequently, a review of substance initiation trends across a larger range of years is especially advised for this age group. See Section B.4.1 in Appendix B of the 2013 national findings report for further discussion of data on trends for past year initiates aged 26 to 49 (CBHSQ, 2014d).
3.4.2.1 Initiation of Use of Tobacco Through Methamphetamine
For cigarettes, smokeless tobacco, cigars, alcohol, marijuana, cocaine, crack cocaine, heroin, hallucinogens, inhalants, and methamphetamine, past year initiation of a given substance in the past year can be viewed as an indicator variable defined as follows:
where (MM/DD/YYYY)Interview denotes the month, day, and year of the interview, and (MM/DD/YYYY)First Use of Substance denotes the date of first use. The total number of past year initiates can be used in the estimation of different percentages. For these substances, denominators for the percentages vary according to whether rates are being estimated for (1) all people in the population (or all people in a subgroup of the population, such as people in a given age group), (2) people who are at risk for initiation because they have not used the substance of interest prior to the past 12 months, or (3) past year users of the substance. The 2020 NSDUH detailed tables show all three of these percentages.
3.4.2.2 Initiation of Misuse of Prescription Psychotherapeutic Drugs
Beginning with the 2015 NSDUH, respondents were asked about the initiation of misuse of prescription psychotherapeutic drugs only for the individual prescription drugs they misused in the past 12 months (see Section C in the methodological summary and definitions report for the 2015 NSDUH; CBHSQ, 2016). An important consideration was that asking respondents to recall their first misuse of any prescription drug in an overall category (e.g., pain relievers) required them to think about the prescription drugs available to them when they initiated misuse. However, some of these drugs may no longer have been available when respondents were interviewed.
If respondents since 2015 reported they first misused one or more prescription drugs at an age or in a year and month more than 12 months prior to the interview date, they logically were not past year initiates for misuse of any drug in that psychotherapeutic category (e.g., pain relievers). If respondents reported only past year initiation of the drugs they misused in the past 12 months, they were asked a follow-up question to determine whether they ever misused any drug in that category more than 12 months prior to the interview.60 Therefore, unlike the situation for other substances in NSDUH (see Section 3.4.2.1), respondents’ status as past year initiates of misuse of any prescription drug in an overall category was determined principally through their answers to the relevant follow-up question.
If respondents answered the follow-up question as “yes,” then they were classified as not being past year initiates for the overall category; the affirmative response indicated respondents had misused one or more other drugs in the category more than 12 months ago. Respondents who answered the follow-up question as “no” were classified as past year initiates for the overall category; the negative response indicated these respondents did not misuse any other drug in that category more than 12 months ago. If respondents answered the follow-up question on initiation as “don’t know” or “refused,” then their status as a past year initiate (or not) was resolved through imputation (see Section 2.3.3).
Because of this question structure for identifying respondents who initiated misuse of any psychotherapeutic drug in a given category in the past year, measures of the age and date of first misuse of any psychotherapeutic drug in that category were created only for respondents who were past year initiates. If past year initiates had no missing data for the age, year, and month when they first misused any psychotherapeutic drug in that category, then the age, year, and month of first misuse logically were assigned from the earliest reports.61 If past year initiates did not know or refused to report the age when they first misused some drugs in that category but they reported first misuse of at least one psychotherapeutic drug in the category at an age 1 year younger than their current age, then it nevertheless could be logically inferred that this was the age when these past year initiates first misused any drug in that category. Similarly, if past year initiates did not know or refused to report the year when they first misused some drugs in that category but they reported first misuse of at least one psychotherapeutic drug in the previous calendar year (e.g., 2019 for respondents in the 2020 NSDUH), then it could be logically inferred respondents initiated misuse of any drug in that category in the previous calendar year. If it was not possible to assign a definite age, year, and month of first misuse for a past year initiate based on the respondent’s questionnaire data, then these values were assigned through imputation.
The total number of past year initiates of misuse of any psychotherapeutic drug in a category can be used in the estimation of percentages among (1) all people in the population (or all people in a subgroup of the population, such as those in a given age group) and (2) people who were past year users of the substance. The 2020 NSDUH detailed tables show estimates for these two percentages.
Because of the change in focus beginning with the 2015 NSDUH questions for specific psychotherapeutic drugs from the lifetime to the past year period (see Section 3.4.1), respondents who last misused any prescription psychotherapeutic drug in a category more than 12 months ago may underreport misuse, especially if they were not presented with examples of drugs formerly available by prescription in the United States but were no longer available at the time respondents were interviewed. These respondents who did not report misuse occurring more than 12 months ago would be misclassified as still being “at risk” for initiation of misuse of prescription drugs in that psychotherapeutic category (i.e., people who initiated misuse more than 12 months ago are no longer at risk for initiation). For this reason, the 2020 detailed tables do not show percentages for initiation of misuse of psychotherapeutic drugs among people who were at risk for initiation.
3.4.3 Substance Use Disorders
The NSDUH questionnaire included questions designed to measure dependence on nicotine (i.e., cigarettes). The questionnaire also included questions to measure SUDs for alcohol and illicit drugs.
3.4.3.1 Nicotine Dependence
For nicotine (cigarettes), questions pertaining to dependence were based on the Nicotine Dependence Syndrome Scale (NDSS; Shiffman et al., 1995, 2004) and the Fagerstrom Test of Nicotine Dependence (FTND; Fagerstrom, 1978; Heatherton et al., 1991).
To identify patterns of nicotine (cigarette) dependence within the 2020 NSDUH data, questions measured dependence on nicotine through the use of cigarettes. Respondents were classified as being dependent if they met either the NDSS or the FTND classifications for dependence. The 2020 NSDUH contained 19 NDSS questions addressing five aspects of dependence: (1) smoking drive (compulsion to smoke driven by nicotine craving and withdrawal), (2) nicotine tolerance, (3) continuous smoking, (4) behavioral priority (preferring smoking over other reinforcing activities), and (5) stereotypy (fixed patterns of smoking). The 2020 NSDUH contained two mutually exclusive questions (DRCGE19a and DRCGE19b) addressing the FTND measure of dependence. These questions ask respondents who reported smoking cigarettes in the past month if the first cigarette they smoked was within 30 minutes of waking up on the days they smoked.
3.4.3.2 Substance Use Disorders for Alcohol and Illicit Drugs
Beginning with the 2020 NSDUH, SUD estimates for alcohol and illicit drugs were based on the criteria in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5; American Psychiatric Association [APA], 2013). Illicit drugs included marijuana, cocaine, heroin, hallucinogens, inhalants, methamphetamine, and the misuse of prescription psychotherapeutic drugs (i.e., pain relievers, tranquilizers, stimulants, and sedatives). Prior to the 2020 NSDUH, SUD estimates for alcohol and illicit drugs were based on criteria in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV; APA, 1994).62
The NSDUH instrument included items asking about SUD symptoms related to the use of a specific substance (or category of substances, such as hallucinogens) in the past 12 months. For methamphetamine, the questions were patterned after questions for cocaine use disorder and were separate from questions for SUD symptoms related to the misuse of prescription stimulants.
Although DSM-5 assesses many of the same criteria as in the DSM-IV, it does not use the diagnoses of dependence and abuse. The DSM-5 SUD diagnosis requires the presence of two or more of the following criteria (as measured in the 2020 NSDUH) in a 12-month period (see Section 3.4.3.3 for comparison of SUD questions for 2020):
- The substance is often taken in larger amounts or over a longer period than intended.
- There is a persistent desire or unsuccessful efforts to cut down or control substance use.
- A great deal of time is spent in activities necessary to obtain the substance, use the substance, or recover from its effects.
- There is craving, or a strong desire or urge to use the substance.
- There is recurrent substance use resulting in a failure to fulfill major role obligations at work, school, or home.
- There is continued substance use despite having persistent or recurrent social or interpersonal problems caused by or exacerbated by the effects of the substance.
- Important social, occupational, or recreational activities are given up or reduced because of substance use.
- There is recurrent substance use in situations in which it is physically hazardous.
- Substance use is continued despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by the substance.
- There is a need for markedly increased amounts of the substance to achieve intoxication or the desired effect, or markedly diminished effect with continued use of the same amount of the substance (i.e., tolerance).
- There are a required number of withdrawal symptoms that occur when substance use is cut back or stopped following a period of prolonged use.63,64 (However, the withdrawal criterion does not apply to hallucinogen use disorder or inhalant use disorder; neither of these substances have a withdrawal syndrome associated with discontinued use.)
Questions were added to the 2020 NSDUH questionnaire to assess craving, marijuana withdrawal, and tranquilizer withdrawal. Craving and marijuana withdrawal were not assessed in prior surveys because they were not part of the DSM-IV SUD criteria. Although tranquilizer withdrawal was part of the DSM-IV criteria, this symptom had not been assessed in prior NSDUHs because some substances previously included in the questionnaire that were classified as tranquilizers do not have a withdrawal component.
Criteria used to determine whether a respondent was asked the SUD questions for alcohol or illicit drugs during the interview included the questions establishing whether respondents used a given substance in the past year (or misused pain relievers, tranquilizers, stimulants, or sedatives in that period); the frequency of substance use questions (for alcohol and marijuana only); questions about the use of cocaine, heroin, or methamphetamine with a needle in the past year; and questions about smoking or sniffing heroin in the past year. Missing or incomplete responses from the respective substance use sections for the most recent use (or misuse) of these substances and the frequency of substance use questions were imputed. Missing or incomplete responses were not imputed for the use of cocaine, heroin, or methamphetamine with a needle or for smoking or sniffing heroin. The questionnaire logic allowed some respondents to be asked the SUD questions for cocaine, heroin, or methamphetamine even if they did not report past year use when they were asked previously about their most recent use of cocaine, crack cocaine, or heroin. Also, responses to the SUD questions for alcohol or marijuana may be inconsistent with responses to the imputed frequency of use variables.
In addition, the severity of an SUD according to DSM-5 criteria is determined by the number of individual criteria recorded as positive for a particular substance (or category of substances).
- People were classified as having an SUD of mild severity if they met two or three SUD criteria for that substance.
- People were classified as having an SUD of moderate severity if they met four or five SUD criteria for that substance.
- People were classified in the severe SUD category if they met six or more SUD criteria for that substance.
Variables for SUD severity also were created for the 2020 NSDUH according to these definitions. However, published estimates for SUD severity outcomes were not presented in reports or tables for the 2020 NSDUH.
3.4.3.3 Comparison of DSM-5 and DSM-IV SUD Criteria
This section discusses differences in SUD definitions in greater detail based on the DSM-5 criteria (published in NSDUH tables and reports for 2020) and the DSM-IV criteria (published before the 2020 NSDUH). As noted previously, people were classified under the DSM-5 criteria as having an SUD for a given substance if they had two or more of the criteria for that substance. In contrast, people were classified as having an SUD based on the DSM-IV criteria according to whether they met criteria for dependence or abuse. People were classified as having dependence if they had three or more of the DSM-IV dependence criteria for a given substance. People were classified as having abuse if they did not meet criteria for dependence but had one or more of the abuse criteria. The 2019 methodological summary and definitions report (CBHSQ, 2020c) provides a detailed list of specific dependence and abuse criteria.
Specific criteria to assess whether someone has an SUD also differ between DSM-5 and DSM-IV.
- DSM-5 (but not DSM-IV) includes a criterion for whether someone had a strong craving or urge to use the substance.
- DSM-5 (but not DSM-IV) includes a withdrawal criterion for cannabis (i.e., marijuana).
- DSM-IV includes a criterion about whether use of a substance caused them to do things that repeatedly got them in trouble with the law. This “legal troubles” criterion for DSM-IV is not included in the SUD criteria for DSM-5.
Table 3.8 also summarizes differences between the former DSM-IV SUD criteria and the DSM-5 criteria. In addition, the withdrawal criterion for DSM-5 and DSM-IV includes use of a substance (or a related substance) to avoid withdrawal symptoms or to make them go away, in addition to people experiencing a required number of withdrawal symptoms when they cut back on or stopped using a substance following a period of prolonged use. However, NSDUH SUD questions based on the DSM-IV criteria asked only if people had the required number of withdrawal symptoms when they cut back on or stopped using a specific substance (e.g., alcohol).
Preliminary analyses of 2020 data suggested that these differences would yield higher SUD estimates in 2020 based on the DSM-5 criteria. SAMHSA concluded that the change from DSM-IV to DSM-5 criteria for estimating SUD would lead to breaks in the comparability of 2020 SUD estimates with estimates from prior years. Consequently, tables and reports for the 2020 NSDUH present SUD estimates only for 2020.
3.4.3.4 Clinical Validation Study
The Clinical Validation Study (CVS) was conducted in early 2020 to assess NSDUH SUD questions that were revised to be consistent with the DSM-5 criteria.
NSDUH respondents were assigned to receive the DSM-5 SUD questions or the DSM-IV SUD questions from the 2019 survey plus supplemental questions about additional DSM-5 criteria not covered by the DSM-IV questions (i.e., craving, marijuana withdrawal, and tranquilizer withdrawal). Otherwise, respondents who received the DSM-5 SUD questions completed the same sections in the same order as other NSDUH interview respondents. Respondents who received the DSM-5 SUD questions are subsequently referred to as “CVS respondents.” Respondents who received the DSM-IV SUD questions and additional questions in the emerging issues section are referred to as “non-CVS respondents.”
Table 3.8 summarizes differences in the questions that CVS and non-CVS respondents received. As discussed previously, for example, questions were added to the emerging issues section of the questionnaire for non-CVS respondents so that data from non-CVS respondents also could be used to create DSM-5 SUD estimates for the full sample. The DSM-IV criterion for legal troubles also was included at the end of the SUD questions for CVS respondents so that data from CVS respondents could be used to create DSM-IV SUD estimates for the full sample.
The sample was planned to be a probability subsample of respondents aged 12 or older during the first 6 months of 2020. However, data collection (including recruitment into the CVS) was suspended on March 16, 2020, because of the COVID-19 pandemic. This suspension of data collection resulted in an approximate halving of the sample size for the CVS. Of the respondents selected for the CVS, slightly more than 500 agreed to participate in the clinical follow-up interview, and clinical interviews were completed with approximately 400 of these respondents.
3.4.3.5 SUD Data for Specific Substances
Beginning with the 2015 NSDUH, missing values in the DSM-IV SUD data for hallucinogens, inhalants, methamphetamine, and prescription psychotherapeutic drugs (i.e., pain relievers, tranquilizers, stimulants, or sedatives) were replaced using the modified predictive mean neighborhoods (modified PMN) imputation method described in Section 2.3.3. Because SUD estimates using the DSM-5 criteria started a new baseline in 2020, modified PMN was used to impute missing DSM-5 SUD data for alcohol and all illicit drugs beginning in 2020. Consequently, the individual SUD as well as the composite measure variables were not subject to the kinds of potential biases due to missing data described in Section 3.3.2.
Imputation of the SUD data reflected imputation of the variables from the corresponding substance use sections for the most recent use of alcohol, marijuana, cocaine, heroin, hallucinogens, inhalants, and methamphetamine, or the misuse for prescription psychotherapeutic drugs. For example, if the edited variable for the most recent use of any hallucinogen indicated use at some point in the respondent’s lifetime and the respondent was imputed to be a past year user, then the SUD outcomes for hallucinogens also were imputed. In addition, if the edited variable for the most recent use of any hallucinogen indicated use in the past 30 days or more than 30 days ago but within the past 12 months, the respondent reported fewer than two SUD symptoms for hallucinogens, and the number of individual hallucinogen use disorder questions with missing data was sufficient to potentially classify the respondent as having a past year hallucinogen use disorder, then modified PMN was used to determine the respondent’s final hallucinogen use disorder outcome. However, individual SUD variables for a given substance were not imputed, only the final SUD and severity outcomes. If the edited variable for the most recent use of any hallucinogen indicated use more than 12 months ago, then no imputation was necessary for hallucinogen use disorder.
For alcohol and marijuana, respondents were asked the SUD questions if they reported substance use on more than 5 days in the past year or if they reported any substance use in the past year but did not report their frequency of past year use (i.e., they had missing frequency data). Therefore, inconsistencies could occur where respondents could be classified with an SUD but their imputed frequency of use variable indicated use on 5 or fewer days in the past year. For alcohol, for example, about 23,000 respondents reported past year alcohol use in 2020.65 Of these, fewer than 50 respondents had missing frequency data and were asked the alcohol use disorder questions, but their final imputed frequency of use indicated they used alcohol on 5 or fewer days in the past year.
For methamphetamine, cocaine, and heroin, respondents were asked the respective SUD questions if they reported past year use in the corresponding substance use sections or if they reported use in the past year in the special drugs section (i.e., use of methamphetamine, cocaine, or heroin with a needle in the past year or smoking or sniffing of heroin in the past year). Thus, the questionnaire logic allowed some respondents to be asked the SUD questions for these drugs even if they did not report past year use when they were asked previously about their most recent use of methamphetamine, cocaine, crack cocaine, or heroin. For cocaine, for example, about 800 respondents in 2020 were asked the questions about cocaine use disorder because they reported past year use when asked directly about their most recent use of cocaine or crack. Fewer than 10 additional respondents were asked these questions because they reported past year use of cocaine with a needle in the special drugs section despite not having previously reported past year use of cocaine or crack.
3.4.3.6 Aggregate Substance Use Disorder Measures
Aggregate SUD measures are included in NSDUH tables, reports, and data files that combine data from multiple SUD measures. These aggregate measures include opioid use disorder (included since the 2016 NSDUH), tranquilizer or stimulant use disorder (included since the 2018 NSDUH), and central nervous system (CNS) stimulant use disorder (beginning with the 2020 NSDUH). Respondents were classified as having a past year use disorder for an aggregate measure if they had a past year use SUD for one or more of the contributing SUD measures.
- Respondents were classified as having an opioid use disorder if they had a heroin use disorder, a pain reliever use disorder, or both in the past year.
- Respondents were classified as having a prescription tranquilizer or sedative use disorder if they had a prescription tranquilizer use disorder, a prescription sedative use disorder, or both in the past year.
- Respondents were classified as having a CNS stimulant use disorder if they had one or more of the following disorders in the past year: cocaine use disorder, methamphetamine use disorder, or prescription stimulant use disorder.
The SUD criteria for specific substances were described previously in Section 3.4.3.2. Respondents were not counted as having an SUD for these aggregate measures if they did not meet the full SUD criteria individually for any of the contributing substances. For example, respondents who met one criterion for heroin use disorder and one criterion for pain reliever use disorder were not classified as having opioid use disorder, regardless of whether the number of discrete symptoms across the heroin and pain reliever use disorders summed to two or more.
3.4.4 Need for Services for Substance Use and Mental Health Issues
3.4.4.1 Need for Substance Use Treatment
In 1998, the Office of National Drug Control Policy (ONDCP) convened an interagency workgroup to discuss options for estimating the need for treatment as it applied to illicit drug use.66 In this meeting, it was established that treatment need could be defined by the presence of an SUD for illicit drugs. However, one concern with this definition was a large number of people who received treatment may not meet the criteria for an illicit drug use disorder. Therefore, this workgroup also established that those who received treatment at a specialty facility should also be classified as needing treatment, regardless of whether they met the criteria for an illicit drug use disorder. Several years after this decision, SAMHSA convened an external expert consultant group to recommend a definition of treatment need for alcohol use. Similar to the illicit drug use treatment need definition, alcohol use treatment need was defined as the presence of an alcohol use disorder or the receipt of treatment at a specialty facility for an alcohol use problem in the past 12 months. The term “specialty facility” is defined below and in the glossary in Appendix A of this report.
Based on the recommendations of the interagency workgroup and the external expert consultant group, the need for substance use treatment is defined for NSDUH according to whether people were classified as needing treatment in the past year for a substance use problem (alcohol use, illicit drug use, or both). Respondents were classified as needing substance use treatment if they met either of the following criteria:
- presence of an SUD in the past year for alcohol or illicit drugs (see Section 3.4.3); or
- receipt of treatment at a specialty facility (i.e., drug and alcohol rehabilitation facility [inpatient or outpatient], hospital [inpatient only], or mental health center) in the past year for the use of alcohol or illicit drugs (or both).
Respondents who reported lifetime use of alcohol or illicit drugs also were asked whether they received substance use treatment in the past year at (1) an emergency room, (2) a private doctor’s office, (3) a prison or jail, (4) a self-help group (e.g., Alcoholics Anonymous or Narcotics Anonymous), or (5) some other place.67 The first four of these additional locations were not considered to be specialty substance use treatment facilities. As discussed in Section 3.4.15, questions that were added to the 2020 NSDUH questionnaire in Quarter 4 for the receipt of virtual (telehealth) services were not associated with a specific provider, location, or facility type. Therefore, receipt of these services could not be grouped into the NSDUH measure for substance use treatment at a specialty facility. Reports of treatment in some other place were considered to be treatment in specialty substance use treatment facilities only if respondents specified a location corresponding to one of the specialty treatment facilities mentioned above.
Respondents who used alcohol or illicit drugs in the past year were classified as not needing substance use treatment if they did not meet criteria for having an SUD in the past year and they did not report receipt of treatment in the past year at a specialty facility. Beginning in 2020, respondents had no missing SUD data (see Sections 2.3.3.1 and 3.4.3.5).
However, respondents who were not classified as having an SUD in the past year could have missing data for whether they received substance use treatment at a specialty facility in the past 12 months. Respondents who had missing information for whether they received any treatment in their lifetime or in the past 12 months for their use of alcohol or illicit drugs were not asked the questions for receipt of substance use treatment at a specialty facility in the past 12 months and were classified as not having received treatment at a specialty facility in the past 12 months; if these respondents did not have an SUD in the past year, then they were classified as not needing substance use treatment. If respondents were not classified as having an SUD in the past year but they reported receiving treatment at a specialty facility in that period, then follow-up questions on the receipt of treatment in a given specialty location for the use of alcohol only, illicit drugs only, or both alcohol and illicit drugs were used to establish whether respondents needed treatment specifically for their use of alcohol or for their use of illicit drugs. If these respondents had missing information for receipt of treatment at a specialty facility in the past 12 months for their use of alcohol only, illicit drugs only, or both alcohol and illicit drugs, it could nevertheless be established that they needed treatment for their use of either alcohol or illicit drugs. Section 3.3.2 discusses the potential bias in estimates because of missing data.
3.4.4.2 Perceived Need for Substance Use Treatment
NSDUH respondents aged 12 or older who used alcohol or illicit drugs68 in their lifetime and reported in the drug treatment section of the questionnaire they did not receive substance use treatment in the past 12 months were asked whether they needed treatment for their use of alcohol or illicit drugs. Respondents who reported they received substance use treatment in the past 12 months were asked whether they needed additional treatment for their use of alcohol or illicit drugs. Respondents who reported they needed treatment or additional treatment in the past 12 months also were asked whether they made an effort to get treatment. If NSDUH respondents reported they did not receive treatment for their alcohol use or illicit drug use in the past 12 months but they needed treatment, they were asked to report the reasons they did not receive treatment. Similarly, respondents who needed additional treatment were asked to report the reasons for not receiving additional treatment.
This information is used in tables and reports to identify the percentage of people with an SUD who did not receive treatment at a specialty facility in the past year69 but nevertheless perceived they needed treatment. In addition, estimates are included in NSDUH reports and tables for whether people made an effort to get treatment if they had an SUD and perceived a need for treatment. As for the need for substance use treatment, missing data for whether respondents needed treatment for their use of alcohol or illicit drugs were handled as though respondents did not perceive a need for treatment; see Section 3.3.2 for a discussion of the potential bias in estimates because of this assumption.
3.4.4.3 Need for Mental Health Services
Unlike the need for substance use treatment, NSDUH does not have an overall measure for whether people aged 12 or older needed mental health services in the past year because mental health questions differ for adults aged 18 or older and for youths aged 12 to 17. Also, there is no behavioral health consensus on how best to define the need for mental health services. Therefore, a definition parallel to the one for the need for substance use treatment may not be appropriate for mental health services. NSDUH reports and tables present estimates of the numbers and percentages of adults aged 18 or older with AMI or SMI who received mental health services in the past year (see Section 3.4.7). NSDUH reports and tables also present estimates for youths and adults with a past year MDE who received treatment for depression in the past year (see Section 3.4.8). Respondents with missing data for whether they received mental health services in the past year or whether they had an MDE in the past year were excluded from the analyses (see Section 3.3.2). Also, estimates in 2020 for the use of mental health services among adults with AMI or SMI and treatment for depression among adults used the break-off analysis weight described in Sections 2.3.4.2, 3.4.7.12, and 3.4.8.
3.4.4.4 Perceived Need for Mental Health Services
Questions in NSDUH about the perceived need for mental health services from the adult mental health utilization section were asked only of adults aged 18 or older. All adult respondents were asked whether they needed mental health treatment or counseling at any time in the past 12 months but did not get it, regardless of whether they reported receiving some type of mental health care in that period. Adults who reported they needed mental health care but did not get it also are asked to report the reasons they did not receive care. Thus, adults who received some type of mental health service in the past 12 months could still report a perceived need for services they did not receive. Adults who received mental health services in the past 12 months also could have felt they had some unmet need either before or after receiving the care.
Adults with missing data for whether they perceived a need for mental health care but did not get it or who had missing data for their reasons for not receiving mental health care were excluded from the analyses (see Section 3.3.2). In addition, 2020 estimates for the perceived unmet need for mental health services among all adults used the main person-level analysis weights that were described in Section 2.3.4. Estimates in 2020 for adults’ perceived unmet need for mental health services used the person-level break-off analysis weights described in Section 2.3.4.2 if these estimates used data from sections listed in Table 2.5 that were affected by break-offs (e.g., perceived unmet need for mental health services among adults with AMI or SMI).
3.4.5 Definition of County Type
County type is based on the “Rural-Urban Continuum Codes”70 developed by the U.S. Department of Agriculture.71 A county type measure was used starting with the 1999 NHSDA72 and was based on the 1993 Rural-Urban Continuum Codes. For the 2002 to 2014 NSDUHs, the county type measure was based on the 2003 Rural-Urban Continuum Codes. Starting with the 2015 NSDUH, the 2013 Rural-Urban Continuum Codes have been used. The county type measures for 2015 and later years are defined using the 2013 Rural-Urban Continuum Codes and are not comparable with the county type measures from the 2002 to 2014 NSDUHs because of the use of different census data and changes to the statistical area definitions. Because counties are defined for all NSDUH respondents, the county type measures did not have missing data.
To create the 2013 Rural-Urban Continuum Codes, all U.S. counties and county equivalents were first grouped according to their official metropolitan-nonmetropolitan status (i.e., statistical area definitions), as determined by the Office of Management and Budget (OMB) in February 2013. This grouping distinguished metropolitan counties by the population size of their metropolitan area and nonmetropolitan counties by their degree of urbanization and adjacency to a metropolitan area. The OMB determined current metropolitan status by applying population and worker commuting criteria to the results of the 2010 census and the 2006-2010 American Community Survey (ACS). No major changes were made in either the metropolitan-nonmetropolitan or urban-rural criteria between 2000 and 2010. However, the decennial census long form was eliminated in 2010, and the OMB used 5-year average commuting flow data from the 2006-2010 ACS rather than a point-in-time estimate to delineate metropolitan and micropolitan areas.
Nonmetropolitan counties in the three urban-sized categories were further subdivided by whether the county was adjacent to one or more metropolitan areas. A nonmetropolitan county was defined as adjacent if it physically adjoined one or more metropolitan areas and had at least 2 percent of its employed labor force commuting to central metropolitan counties. Nonmetropolitan counties not meeting these criteria were classed as nonadjacent. The 2006-2010 ACS commuting flow data were also used to compute adjacency for the 2013 Rural-Urban Continuum Codes.
Metropolitan and nonmetropolitan categories were subdivided into three metropolitan and six nonmetropolitan categories, resulting in a nine-part county codification.
- Large metropolitan statistical areas (MSAs) (large metropolitan) have a total population of 1 million or more.
- Small MSAs (small metropolitan) have a total population of fewer than 1 million.
- Nonmetropolitan counties were classified according to the aggregate size of their urban population. Nonmetropolitan areas include counties in micropolitan statistical areas and counties outside of both metropolitan and micropolitan statistical areas and are classified as follows:
- “urbanized,”
- “less urbanized,” and
- “completely rural.”
The OMB defined nonmetropolitan counties according to (1) the size of the population in urbanized areas within the county (i.e., a population of 20,000 or more in urbanized areas, a population of at least 2,500 but fewer than 20,000 in urbanized areas, or a population of fewer than 2,500 in urbanized areas), and (2) whether these counties were adjacent or not adjacent to a metropolitan area. For NSDUH, these nonmetropolitan categories were categorized as “urbanized,” “less urbanized,” and “completely rural.” The terms “urbanized,” “less urbanized,” and “completely rural” for counties are not based on the relative proportion of the county population in urbanized areas but rather are based on the absolute size of the population in urbanized areas. For example, some counties classified as “less urbanized” had over 50 percent of the county population residing in urbanized areas, but this percentage represented fewer than 20,000 people in the county.
3.4.6 Effects of Questionnaire Changes Prior to 2015 on Mental Health Measures
Changes were made to the mental health questions in the 2008 and 2009 NSDUH questionnaires that were relevant to the estimation of mental health issues and the use of mental health services. These changes are summarized because mental health measures in 2020 NSDUH reports and tables include data from these years. The specific changes in 2008 and 2009 are described below.
- For adults aged 18 or older, changes were made to the K6 questions for measuring serious psychological distress (SPD). In 2007, a single set of six K6 items asked adult respondents to report how often they experienced certain emotions or feelings during the 1 month in the past 12 months they were the most depressed, anxious, or stressed. In 2008, adult respondents first were asked about these feelings in the past 30 days. If there was a month in the past 12 months when they felt more depressed, anxious, or emotionally stressed than they felt during the past 30 days, they then were asked the same K6 items about this month as well. The K6 questions for the past 30 days and past 12 months were used in models predicting AMI or SMI (see Section 3.4.7).
- For adults aged 18 or older, a split-sample study was embedded within the 2008 NSDUH mental health section of the CAI, such that a reduced set of questions from the World Health Organization Disability Assessment Schedule (WHODAS) or the Sheehan Disability Scale (SDS) were randomly assigned to respondents to assess impairment because of psychological distress. The WHODAS questions were retained for use in the 2009 NSDUH and future models predicting AMI or SMI. These SDS items for impairment because of psychological distress were no longer included after 2008, but they continued to be included for estimating severe impairment because of an MDE (see Section 3.4.8).
- For youths aged 12 to 17, a total of five questions in the youth mental health service utilization (YMHSU) section in 2008 were no longer included in 2009. These questions were replaced with seven questions asking about receipt of mental health services in the education and justice system sectors.
For the first change, the past year K6 scores since 2008 have been created for each adult aged 18 or older based on responses to items regarding either the past 30 days (if adults said they did not have any other worse month) or the worst month in the past 12 months. This change in questionnaire structure was evaluated to determine whether this change may have affected K6 scores and estimates of SPD created from the K6 items for the worst month in the past year.
The remaining changes to questions between survey years also could have affected how respondents answer questions in subsequent sections (i.e., context effects). A context effect may be said to take place when the response to a question is affected by information not part of the question itself. For example, the content of a preceding question may affect the interpretation of a subsequent question. A respondent also may answer a subsequent question in a manner consistent with responses to a preceding question if the two questions are closely related to each other.73 Therefore, these changes were evaluated for possible context effects.
3.4.6.1 Effects of Changes to the Questions for Adults
For adults aged 18 or older, estimates of past year K6 scores and the percentage of adults with SPD based on the entire 2008 sample, as well as the WHODAS and SDS subsamples, were compared with estimates based on 2007 data. Significant differences in the mean past year K6 scores were observed between 2008 and 2007, thus suggesting a lack of comparability in SPD estimates between the 2 years. Across each of the six items forming the past year K6 score, estimates of adults reporting they had a given problem “none of the time” (e.g., “how often felt restless in worst month”) were higher in 2008 based on the full sample of adults compared with the estimates for 2007. The estimate of past year SPD was slightly lower from the full sample of adults in 2008 than in 2007.
In the 2008 NSDUH, the psychological distress module that previously included only a set of six past year K6 items was renamed the mental health module. This module was expanded to include a version of the K6 that included items on the past 30 days (which resulted in some changes to the past year K6 items), followed by the WHODAS or the SDS, as well as items on suicidal thoughts and behavior. The split-sample design in 2008 for adults (item 2 in Section 3.4.6) affected the reporting of MDE in the adult depression module (following the mental health module), depending on whether adult respondents received the WHODAS or the SDS. Both lifetime and past year MDE estimates among the adults based on the WHODAS half sample were lower than corresponding estimates from 2007. In turn, lifetime and past year MDE estimates based on the entire sample in 2008 were lower than corresponding estimates from 2007. However, estimates of lifetime and past year MDE based on the SDS half sample in 2008 were not significantly different from the estimates in 2007. Also, the estimate of past year MDE in 2008 based on the WHODAS half sample was lower than the estimate based on the SDS half sample.
Therefore, CBHSQ decided to publish estimates of adult MDE in 2008 based on the half sample of adults who received the WHODAS because CBHSQ decided to retain the WHODAS in subsequent surveys. However, subsequent adjustment procedures were developed for adult MDE from the SDS half sample to allow data from all adult respondents in 2008 to be used for estimating MDE among adults. These adjustment procedures are described further in this report in Section 3.4.8.
Administration of the WHODAS or SDS in 2008 did not appear to differentially affect responses to the questions added in 2008 for suicidal thoughts and behavior among adults. Therefore, further investigation was not done to examine the effects on estimates of suicidal thoughts and behavior in 2009 due to the removal of the SDS items.
3.4.6.2 Effects of Changes to the Questions for Youths
The changes to the YMHSU section (item 3) in 2009 could have affected how adolescents answered the items at the beginning of the adolescent depression section (i.e., due to context effects). The adolescent depression section follows the YMHSU section for youths. In turn, changes in youths’ answers to these introductory adolescent depression items could affect estimates of adolescent MDE.
Adolescents aged 12 to 17 could be asked up to three questions (YDS21, YDS22, and YDS23) to determine whether they should be asked further questions about lifetime and past year MDE. All adolescents were asked question YDS21: “Have you ever in your life had a period of time lasting several days or longer when most of the day you felt sad, empty, or depressed?” Those who did not answer question YDS21 as “yes” then were asked question YDS22: “Have you ever had a period of time lasting several days or longer when most of the day you felt very discouraged or hopeless about how things were going in your life?” Youths who did not answer either question YDS21 or YDS22 as “yes” then were asked question YDS23: “Have you ever had a period of time lasting several days or longer when you lost interest and became bored with most things you usually enjoy, like work, hobbies, and personal relationships?” Any adolescent who gave an affirmative answer in questions YDS21, YDS22, or YDS23 then was administered additional depression-related items also used to determine whether adolescents had a lifetime and past year MDE.
The effects of these changes to the YMHSU section on subsequent reports in the adolescent depression section were investigated using data from the first 6 months of the 2009 NSDUH. This analysis sought to determine whether changes in the YMHSU section affected responses to the first three adolescent depression questions and the lifetime and past year MDE estimates. To assess whether any difference in estimates between 2008 and 2009 could be due to more than just true changes in the population, comparisons also were carried out between estimates in consecutive years beginning in 2005. For consistency with the 2009 data, comparisons were limited to the first 6 months of NSDUH data from other survey years.
The changes to the YMHSU section in 2009 did not appear to affect estimates for the variables based on the lead adolescent depression questions or estimates of adolescent MDE between 2008 and 2009. None of the differences in estimated responses to the three lead adolescent MDE items or estimates of adolescent lifetime and past year MDE between 2008 and 2009 was statistically significant. No apparent trend was observed between 2005 and 2009 for the lifetime and past year MDE estimates or for the variable corresponding to question YDS23. Therefore, it was determined the youth depression estimates could continue to be compared between 2009 and prior years.
3.4.7 Estimation of Serious and Other Levels of Mental Illness
3.4.7.1 Background
The 1992 Alcohol, Drug Abuse, and Mental Health Administration Reorganization Act created SAMHSA and required the new organization to develop a definition and methodology for estimating SMI among adults for use by states in developing their plans for use of block grant funds distributed by SAMHSA. A technical advisory group convened by SAMHSA was tasked with developing a definition of SMI, which was published in the Federal Register in 1993 (SAMHSA, 1993):
Pursuant to Section 1912(c) of the Public Health Service Act, as amended by Public Law 102-321, “adults with serious mental illness” are defined as the following:
- Individuals aged 18 and over, who currently or at any time during the past year, have had diagnosable mental, behavioral, or emotional disorder of sufficient duration to meet diagnostic criteria specified within DSM-III-R that has resulted in functional impairment, which substantially interferes with or limits one or more major life activities.
- These disorders include any mental disorder (including those of biological etiology) listed in DSM-III-R or their ICD-9-CM equivalent (and subsequent revisions), with the exception of DSM-III-R “V” codes, substance use disorders, and developmental disorders, which are excluded unless they co-occur with other diagnosable serious mental illness.
- All of these disorders have episodic, recurrent, or persistent features; however, they vary in terms of severity or disabling effects. Functional impairment is defined as difficulties that substantially interfere with or limit role functioning in one or more major life activities including basic daily living skills (e.g., eating, bathing, dressing); instrumental living skills (e.g., maintaining a household, managing money, getting around the community, taking prescribed medication); and functioning in social, family, and vocational/educational contexts.
- Adults who would have met functional impairment criteria during the referenced year without benefit of treatment or other support services are considered to have serious mental illness.
In NSDUH reports prior to 2004, the K6 psychological distress scale was used to measure SMI. In 2004, yearly estimation of SMI ceased temporarily because of concerns about the validity of using only the K6 distress scale to measure SMI without including a functional impairment scale (see Section B.4.4 of Appendix B in the 2004 NSDUH national findings report [Office of Applied Studies (OAS), 2005] for a discussion). In December 2006, a new technical advisory group was convened by SAMHSA’s Office of Applied Studies (which later became CBHSQ) and the Center for Mental Health Services to solicit recommendations for data collection strategies to address SAMHSA’s legislative requirements.
Although the technical advisory group recognized the ideal way to estimate SMI in NSDUH would be to administer a clinical diagnostic interview annually to all 45,000 adult respondents,74 this approach was not feasible because of constraints on the interview time and the need for trained mental health clinicians to conduct the interviews. Therefore, the approach recommended by the technical advisory group and adopted by SAMHSA for NSDUH was to utilize short scales in the NSDUH interview to separately measure psychological distress and functional impairment for use in a statistical model predicting whether a respondent had mental illness. To accomplish this, SAMHSA’s CBHSQ initiated a Mental Health Surveillance Study (MHSS) in 2007 as part of NSDUH to develop and implement methods to estimate SMI. Models using the short scales for psychological distress and impairment to predict mental illness status were developed from a subsample of adult respondents who had completed the NSDUH interview and were administered a clinical psychological diagnostic interview. For the clinical interview data, people were classified as having SMI if they had a diagnosable mental, behavioral, or emotional disorder in the past 12 months, other than a developmental disorder or SUD, that met DSM-IV criteria (APA, 1994) and resulted in substantial functional impairment. This estimation methodology was implemented in the 2008 NSDUH.
3.4.7.2 Historical Summary of the 2008 Model
A randomly selected subsample of approximately 1,500 adults in 2008 who had completed the NSDUH interview was recruited for a follow-up clinical interview consisting of a diagnostic assessment for mental disorders.75 Also, in order to determine the optimal scale for measuring functional impairment in NSDUH, a split-sample design was incorporated into the full 2008 NSDUH data collection. Roughly half of the adult respondents were assigned to receive an abbreviated eight-item version of the WHODAS (Novak et al., 2010), and the other half were assigned to receive the SDS (Leon et al., 1997).
Weighted logistic regression models predicting mental illness were developed for each half sample using the data from the subsample of MHSS respondents. The short scales (the K6 in combination with the WHODAS or the K6 in combination with the SDS) were used as predictors in models of mental illness assessed via the clinical interviews. The model parameter estimates then were used to predict SMI in the full 2008 NSDUH sample. For more detailed information on the 2008 MHSS design and analysis, see Colpe et al. (2009) and OAS (2009a). Information about the 2008 model is available in Appendix B of the 2012 mental health findings report (CBHSQ, 2013b).
Based on an analysis of the 2008 MHSS data, it was determined the WHODAS was the better predictor of SMI and this scale would be used in combination with the K6 scale to predict SMI. It also was decided the WHODAS would continue to be administered as the sole impairment scale in the 2009 and subsequent NSDUHs (OAS, 2009a). This model, developed using the 2008 data (subsequently referred to as the “2008 model”), was used in the 2008 through 2011 NSDUHs to produce a predicted probability of having SMI for each clinical interview respondent.
Based on the accumulated MHSS clinical data collected from 2008 to 2012, however, SAMHSA determined the 2008 model had some important shortcomings not detected in the original model fitting because of the small number of respondents in the 2008 clinical sample. Specifically, estimates of SMI and AMI among young adults based on the NSDUH main study data and prediction model were higher than the estimates for this age group based on the clinical interview data. In addition, improvements were needed in the weighting procedures for the MHSS clinical data to account better for undercoverage and nonresponse (i.e., because only NSDUH respondents who answered their surveys in English were eligible for the clinical follow-up and because people with mental illness appeared to be more likely to participate in the follow-up). Therefore, using the combined 2008 to 2012 clinical data, SAMHSA fit a more accurate model for the 2012 estimates with revised weights (subsequently referred to as the “2012 model”). In particular, to reduce bias and improve prediction, additional mental health-related variables and an age variable were added in the 2012 model. In addition, to protect against potential coverage and nonresponse error, alternatives for the weights were applied to the clinical sample data for the model development. To provide consistent data for trend assessment, mental illness estimates for 2008 to 2011 were revised using the new 2012 model. The 2012 model has been used for all NSDUH mental illness estimates since 2012.
The next sections describe the instruments and items used to measure the variables employed in the 2012 model. Specifically, the instrument used to measure mental illness in the clinical interviews is described, followed by descriptions of the scales and items in the main NSDUH interviews used as predictor variables in the model (e.g., the K6 and WHODAS total scores, age, and suicidal thoughts).76 Next, procedures for the MHSS clinical interview sampling and weighting and for developing the 2012 model are described. Section 3.4.7.10 discusses SEs for the mental illness estimates based on the 2012 model. Remaining sections discuss miscellaneous issues for the mental illness variables.
3.4.7.3 Clinical Measurement of Mental Illness
Mental illness was measured in the MHSS clinical interviews using an adapted version of the SCID (First et al., 2002) and was differentiated by the level of functional impairment based on the Global Assessment of Functioning (GAF) scale (Endicott et al., 1976).77 Past year disorders assessed through the SCID included mood disorders (e.g., MDE, manic episode), anxiety disorders (e.g., panic disorder, generalized anxiety disorder, posttraumatic stress disorder), eating disorders (e.g., anorexia nervosa), intermittent explosive disorder, and adjustment disorder. In addition, the presence of psychotic symptoms was assessed. SUDs also were assessed, although these disorders were not used to produce estimates of mental illness.
- Respondents were classified as having any mental illness (AMI) if they were determined to have any of the mental disorders assessed in the SCID (not including SUDs), regardless of the level of functional impairment.
- Respondents were classified as having low (mild) mental illness if they had any of the mental disorders assessed in the SCID (not including SUDs), but these disorders resulted in no more than mild impairment, based on GAF scores of greater than 59.
- Respondents were classified as having moderate mental illness if they had any of the mental disorders assessed in the SCID (not including SUDs), and these disorders resulted in moderate impairment, based on GAF scores of 51 to 59.
- Respondents were classified as having serious mental illness (SMI) if they had any of the mental disorders assessed in the SCID (not including SUDs), and these disorders resulted in substantial impairment in carrying out major life activities, based on GAF scores of 50 or below. The SMI diagnosis was used as the response variable in both the 2008 and 2012 prediction models.
The SCID and the GAF in combination were considered to be the “gold standard” for measuring mental illness.
3.4.7.4 K6
The K6 in the main NSDUH interview consists of two sets of six questions in the mental health section asking adult respondents how frequently they experienced symptoms of psychological distress during two different time periods: (1) during the past 30 days and, (2) if applicable, the 1 month in the past year when they were at their worst emotionally. Respondents were asked about the second time period only if they indicated there was a month in the past 12 months when they felt more depressed, anxious, or emotionally stressed than they felt during the past 30 days.
The six questions in the K6 scale for the past month are as follows:
- NERVE30
- During the past 30 days, how often did you feel nervous?
1All of the time
2Most of the time
3Some of the time
4A little of the time
5None of the time
Don’t know/Refused
Response categories are the same for the remaining questions shown below.
- HOPE30
- During the past 30 days, how often did you feel hopeless?
- FIDG30
- During the past 30 days, how often did you feel restless or fidgety?
- NOCHR30
- During the past 30 days, how often did you feel so sad or depressed that nothing could cheer you up?
- EFFORT30
- During the past 30 days, how often did you feel that everything was an effort?
- DOWN30
- During the past 30 days, how often did you feel down on yourself, no good or worthless?
In the 2020 NSDUH data, all adult respondents with item nonresponse for psychological distress items (based on the K6 distress scale) had their scores assigned as zeros. In particular, respondents who reported in the K6 questions they had all six symptoms of psychological distress “none of the time” in the past 30 days or their worst period in the past 12 months (if applicable) were classified as not having psychological distress. Similarly, if respondents answered some of the K6 questions as “don’t know” or “refused” and the remainder as “none of the time” (i.e., with no indication of having symptoms at least a little of the time), then these respondents were classified as not having psychological distress. Of the approximately 30,000 final adult respondents in the 2020 NSDUH, roughly 1,100 had at least one of the six past month K6 item scores missing.78 Of those, about 870 had all six item scores missing, mostly because of break-offs (see Sections 2.3.4.2 and 3.3.3.1). As a result of assigning zeros to the K6 scores when respondents answered the questions as “don’t know” or “refused,” there were no missing values in the 2020 survey for measures of adult SMI and other mental illness measures created from a model using the K6 scores. However, the missing data issues described in Sections 3.3.2 and 3.3.3.1 applied to the K6 scores, especially because of adult respondents who broke off the interview before receiving the K6 questions.
To create a score, the six items (NERVE30, HOPE30, FIDG30, NOCHR30, EFFORT30, and DOWN30) on the K6 scale were recoded from 0 to 4 so that “all of the time” was coded as 4, “most of the time” as 3, “some of the time” as 2, “a little of the time” as 1, and “none of the time” as 0. As noted previously, responses of “don’t know” or “refused” also were coded as 0. Summing across the transformed responses in these six items resulted in a score with a range from 0 to 24.
If respondents were asked about a month in the past 12 months when they felt more depressed, anxious, or emotionally stressed than they felt during the past 30 days, they were asked comparable K6 items for that particular month in the past 12 months. The scoring procedures for these K6 items for the past 12 months were the same as those described previously for the past 30 days. The higher of the two K6 total scores for the past 30 days or past 12 months was used both for MHSS analysis purposes and in the adult respondents’ final data.
An alternative K6 total score was created in which K6 scores of less than 8 were recoded as 0. A score of 8 was recoded as 1, a score of 9 was recoded as 2, and so on, until a score of 24 was recoded as 17. The rationale for creating the alternative past year K6 score was that SMI prevalence typically was extremely low for respondents with past year K6 scores of less than 8, and the prevalence rates started increasing only when scores were 8 or greater. This alternative K6 score was used in both the 2008 and 2012 SMI prediction models.
3.4.7.5 WHODAS
An initial step of the MHSS was to modify the WHODAS for use in a general population survey, including making minor changes to question wording and reducing its length (Novak, 2007). That is, a subset of 8 items was found to capture the information represented in the full 16-item scale with no significant loss of information.
Respondents who were not administered the WHODAS because their total K6 score was zero were assigned a zero value for the individual WHODAS items. This includes respondents who reported in the K6 questions they had all six symptoms of psychological distress “none of the time” in the past 30 days or their worst period in the past 12 months (if applicable) or who answered some of the K6 questions as “don’t know” or “refused” and the remainder as “none of the time” (i.e., with no indication of having symptoms at least a little of the time).
Approximately 6,200 respondents were skipped out of the WHODAS questions in 2020 because the sum of all imputation-revised K6 item scores79 was zero, including scores of zero from more than 800 respondents who broke off the interview before or during the K6 questions. Of these respondents who were skipped out of the WHODAS questions because of a zero total K6 score, slightly more than 5,300 responded to all of the K6 items. Of the approximately 23,700 final adult respondents who were asked the WHODAS questions in the 2020 NSDUH, about 330 had at least one of the eight WHODAS item scores missing, and slightly fewer than 50 had all eight item scores missing. As a result of assigning zeros to the WHODAS scores when respondents answered the questions as “don’t know” or “refused” or because of missing data in the K6 items, there were no missing values in the 2020 survey for measures of adult SMI and other mental illness measures created from a model using the WHODAS scores. However, the missing data issues described in Sections 2.3.4.2, 3.3.2, and 3.3.3.1 applied to the WHODAS scores.
The eight WHODAS items included in the main NSDUH mental health section of the interview were assessed on a 0 to 3 scale, with responses of “no difficulty,” “Don’t know,” and “refused” coded as 0; “mild difficulty” coded as 1; “moderate difficulty” coded as 2; and “severe difficulty” coded as 3. Some items had an additional category for respondents who did not engage in a particular activity (e.g., they did not leave the house on their own). Respondents who reported they did not engage in an activity were asked a follow-up question to determine if they did not do so because of emotions, nerves, or mental health. Those who answered “yes” to this follow-up question were subsequently assigned to the “severe difficulty” category; otherwise (i.e., for responses of “no,” “Don’t know,” or “refused”), they were assigned to the “no difficulty” category. Summing across these codes for the eight responses resulted in a total score with a range from 0 to 24. More information about scoring of the WHODAS can be found in the 2018 NSDUH public use file and codebook (CBHSQ, 2019).
An alternative WHODAS total score was created in which individual WHODAS item scores of less than 2 were recoded as 0, and item scores of 2 to 3 were recoded as 1. The individual alternative item scores then were summed to yield a total alternative score ranging from 0 to 8. Creation of an alternative version of the WHODAS score assumed a dichotomous measure dividing respondents into two groups (i.e., severely impaired vs. less severely impaired) might fit better than a linear continuous measure in models predicting SMI. This alternative WHODAS score was the variable used in both the 2008 and 2012 SMI prediction models.
3.4.7.6 Suicidal Thoughts, MDE, and Age
In addition to the K6 and WHODAS scales, the 2012 model included the following measures as predictors of SMI: (1) serious thoughts of suicide in the past year, (2) having a past year MDE, and (3) age. The first two variables were added to the model to decrease the error rate in the predictions (i.e., the sum of the false-negative and false-positive rates relative to the clinical interview results). A recoded age variable reduced the biases in estimates for particular age groups, especially for 18- to 25-year-olds.
Since 2008, all adult respondents in NSDUH have been asked the following question in the mental health section about serious thoughts of suicide: “At any time in the past 12 months, that is from [DATEFILL] up to and including today, did you seriously think about trying to kill yourself?”80 Definitions for MDE in the lifetime and past year periods were based on questions in the adult depression section and are discussed in Section 3.4.8. For the modeling, missing data from adult respondents for whether they had serious thoughts of suicide or for having a past year MDE were treated as being equivalent to negative responses (i.e., no serious thoughts of suicide or not having a past year MDE).81 The missing data issues described in Section 3.3.2 applied to the handling of the suicide and MDE data in the model.
For respondents aged 18 to 30, an adjusted age was created by subtracting 18 from the respondent’s current age, resulting in values ranging from 0 to 12. For a respondent aged 18, for example, the adjusted age was 0 (i.e., 18 minus 18), and for a respondent aged 30, the adjusted age was 12 (i.e., 30 minus 18). For respondents aged 31 or older, the adjusted age was assigned a value of 12.
3.4.7.7 Sampling and Weighting of the 2012 Model
The target annual respondent sample sizes for the MHSS clinical interviews were 1,500 in 2008 (750 of which received the WHODAS and were used in developing the 2008 model), 500 in 2009 and 2010, and 1,500 in 2011 and 2012. Respondent sample sizes were roughly equal across quarters.
A stratified Bernoulli selection process was used in which all eligible NSDUH respondents were given an independent probability of selection based on their strata. In 2008 and the first two quarters in 2009, stratification was based on K6 scores in an attempt to minimize the variance of the estimate for SMI prevalence. In the last two quarters in 2009, stratification attempted to minimize the variance of the AMI prevalence estimate rather than the variance of the SMI estimate. This change reduced the probability a respondent with an extremely large weight would be selected. Starting from 2010, stratification for the MHSS sample incorporated information on functional impairment levels (WHODAS scores) and age in addition to K6 scores. Younger age groups were undersampled for the MHSS clinical sample to reverse the impact of the oversampling of young adults aged 18 to 25 in the main survey (see Section A.1 in Appendix A in the 2012 NSDUH mental health findings report [CBHSQ, 2013b]). This resulted in a more equally allocated clinical sample by age. More details about the sample design for the MHSS clinical study can be found in the 2012 NSDUH’s sample design report (CBHSQ, 2013a).
Special clinical sample analysis weights were created. Each was the product of the following seven weight components: (1) the NSDUH analysis weight; (2) a coverage adjustment for Hispanics completing the main NSDUH interview in English to account for Hispanics who completed it in Spanish and thus were not eligible for the English-language clinical follow-up interview; (3) the inverse of the selection probability for clinical follow-up; (4) a refusal adjustment to account for NSDUH respondents who were selected for the MHSS but declined to be contacted for the clinical interview; (5) another nonresponse adjustment to account for MHSS nonresponse among NSDUH respondents who had originally agreed to be recontacted for the clinical interview but did not complete the interview; (6) poststratification adjustments to reduce the variance of the resulting estimates by matching the weighted main NSDUH interview sample by age, gender, race/ethnicity, alternative K6 score, alternative WHODAS score, having had serious thoughts of suicide in the past year, and having had an MDE;82 and (7) a yearly scaling factor. The first six weight components were created separately for each year.
Separate sets of analysis weights were computed for (1) MHSS respondents from the 2008 half sample assigned to impairment questions derived from the WHODAS and (2) MHSS respondents from the half sample assigned to the alternative scale for measuring impairment based on the SDS. Only the MHSS respondents from the WHODAS half sample were used in determining and fitting the 2012 model.
The 2012 model was fit by assuming the relationship between SMI and the covariates of the model stayed the same from 2008 through 2012. Because the sample size, sampling allocation, and weight adjustments for the MHSS clinical samples differed across years, gains in statistical efficiency were realized by scaling the weights in each year using the following scaling factors: 12 percent for 2008, 4 percent for 2009, 14 percent for 2010, 35 percent for 2011, and 35 percent for 2012. The scaling factors were determined based on the relative sizes of the estimated variances for estimates of SMI, AMI, and past year MDE made directly from SCID diagnoses.83
3.4.7.8 The 2012 SMI Model
The 2012 SMI prediction model was fit with data from 4,912 WHODAS MHSS respondents from 2008 through 2012. The response variable Y equaled 1 when an SMI diagnosis was positive based on the clinical interview; otherwise, Y was 0. Letting X be a vector of the characteristics attached to a NSDUH respondent and letting the probability this respondent had SMI be 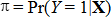 , the 2012 SMI prediction model was as follows:
, the 2012 SMI prediction model was as follows:
where 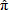 refers to the estimate of the SMI response probability
refers to the estimate of the SMI response probability  .
.
These covariates in equation (1) came from the main NSDUH interview data:
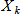 = Alternative Past Year K6 Score: Past year K6 score of less than 8 recoded as 0; past year K6 score of 8 to 24 recoded as 1 to 17.
= Alternative Past Year K6 Score: Past year K6 score of less than 8 recoded as 0; past year K6 score of 8 to 24 recoded as 1 to 17.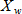 = Alternative WHODAS Score: WHODAS item score of less than 2 recoded as 0; WHODAS item score of 2 to 3 recoded as 1, then summed for a score ranging from 0 to 8.
= Alternative WHODAS Score: WHODAS item score of less than 2 recoded as 0; WHODAS item score of 2 to 3 recoded as 1, then summed for a score ranging from 0 to 8.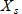 = Serious Thoughts of Suicide in the Past Year: Coded as 1 if “yes”; coded as 0 otherwise.
= Serious Thoughts of Suicide in the Past Year: Coded as 1 if “yes”; coded as 0 otherwise.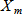 = Past Year MDE: Coded as 1 if the criteria for past year MDE were met (see Section 3.4.8);84 coded as 0 otherwise.
= Past Year MDE: Coded as 1 if the criteria for past year MDE were met (see Section 3.4.8);84 coded as 0 otherwise.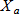 = Adjusted Age: Coded as age minus 18 if aged 18 to 30; coded as 12 otherwise.
= Adjusted Age: Coded as age minus 18 if aged 18 to 30; coded as 12 otherwise.
As with the 2008 model, a cut point probability 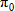 was determined, so that if
was determined, so that if 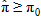 for a particular respondent, then the respondent was predicted to be SMI positive; otherwise, the respondent was predicted to be SMI negative. The cut point (0.260573529) was chosen so that the weighted numbers of false positives and false negatives in the MHSS dataset were as close to equal as possible. The predicted SMI status for all adult NSDUH respondents was used to compute prevalence estimates of SMI.
for a particular respondent, then the respondent was predicted to be SMI positive; otherwise, the respondent was predicted to be SMI negative. The cut point (0.260573529) was chosen so that the weighted numbers of false positives and false negatives in the MHSS dataset were as close to equal as possible. The predicted SMI status for all adult NSDUH respondents was used to compute prevalence estimates of SMI.
A second cut point probability (0.0192519810) was determined so that any respondent with an SMI probability greater than or equal to the cut point was predicted to be positive for AMI, and the remainder were predicted to be negative for AMI. The second cut point was chosen so that the weighted numbers of AMI false positives and false negatives were as close to equal as possible.
Using a combination of the defined mental illness measures, additional levels of mental illness were created based on the 2012 SMI model with the clinical interview data. These additional levels include moderate mental illness (MMI) and mild (low) mental illness (LMI). Clinical interview respondents were classified as having past year MMI if they had serious or moderate mental illness (SMMI; GAF score below 6085) but did not have SMI. Respondents were classified as having past year LMI if they had AMI but did not have SMMI.
Estimates of SMMI were analogously computed in the model with the SMI method; the cut point for SMMI was 0.077686285365. Estimates of LMI and MMI were derived by a process of subtraction. Respondents were classified as belonging to the MMI category if they belonged to the SMMI category but did not belong to the SMI category. Respondents were classified as belonging to the LMI category if they belonged to the AMI category but not to the SMMI category.
Beginning with the 2014 first findings reports, however, estimates for LMI and MMI were replaced with estimates for AMI without SMI; beginning with the 2015 first findings reports, this term was changed to “AMI excluding SMI.” Adults with AMI excluding SMI currently or at any time in the past year have had a diagnosable mental, behavioral, or emotional disorder resulting in less than substantial impairment in carrying out major life activities (see Appendix A in this report). Thus, adults with AMI excluding SMI had either LMI or MMI. Estimates for LMI and MMI also are no longer shown in the detailed tables starting with the 2016 NSDUH. Instead, also starting with the 2016 detailed tables, estimates for mental illness among adults are shown for AMI, SMI, and AMI excluding SMI.
3.4.7.9 Alternative 2012 Model for the SDS Half Sample
In 2008, approximately half of the respondents in the adult NSDUH sample were assigned to receive questions about impairment based on the WHODAS (referred to as the 2008A sample), and the other half were assigned to receive questions based on the SDS (referred to as the 2008B sample). As noted previously, the purpose of this split sample was to determine whether the SDS or WHODAS impairment scale was a better predictor of SMI. The WHODAS scale was identified as the better predictor.
For the clinical interview respondents who had been administered the SDS in the main survey, an alternative SMI model was fit using the complete MHSS dataset of clinical interviews from 2008 through 2012. SMI, AMI, and SMMI estimates were obtained using the same cut point methodology described previously but applied to the alternative model. Mental illness estimates based on the predicted values for the 2008B sample were compared with the ones based on the 2008A sample using the 2012 model described previously. The model-based estimates from the 2008A and 2008B samples were similar, and the predicted values for the two half samples in 2008 were deemed to be comparable. For example, the AMI estimates for the 2008A and 2008B half samples were 17.69 and 17.78 percent, respectively. Therefore, the predicted values from the 2008B sample were combined with predicted values from the complete WHODAS sample for 2008A and for 2009 through 2012.
In fitting the alternative 2012 model for the SDS half sample, weights for the clinical interview respondents who had been assigned to the SDS were developed separately using the same steps as in other years. The 2008 sample of clinical interview respondents who had received WHODAS questions in NSDUH was treated as being equivalent to a sample in a different year. When data from clinical interview respondents were combined from the 2008A, 2008B, 2009, 2010, 2011, and 2012 samples, the 2008A and 2008B weights were each scaled by 6 percent (0.06). Weights for the other years were scaled as described previously.
The modified 2012 SMI prediction model for the SDS half sample was as follows:
All of the covariates in equation (2) appeared in equation (1) as well.
The estimates of the parameters of the models displayed in equations (1) and (2) are given in Table 3.9 shown at the end of this chapter.
3.4.7.10 Standard Errors for Mental Illness Estimates
For this report and the detailed tables, SEs for mental illness estimates (SMI, AMI, and AMI excluding SMI) were computed using the NSDUH dichotomous variable values without taking into account any variance introduced through using a model based on the clinical subsample data. This ignores the added error resulting from fitting the 2012 SMI model, which can be very large (see the 2012 MHSS design and estimation report; CBHSQ, 2014a). These conditional SEs (conditional on the model predictions being correct) are useful when making comparisons across years and across subpopulations (except those involved in modeling) within years because the errors due to model fitting are nearly the same across the estimates being compared.
3.4.7.11 Limitations in Using the Mental Illness Variables in Analyses
There are many advantages to using the cut point methodology described in this section to predict the SMI and AMI status for every adult responding to the NSDUH main survey interview. For some analyses, however, these predicted values should not be used. In particular, these predicted values should not be employed in analyses using the mental illness variables in conjunction with variables used or closely related to variables used in the prediction model. In particular, estimates of SMI or AMI should not be computed using these predicted NSDUH data for mental illness for the following groups of adults: among people with past year or lifetime MDE; among people with past year suicidal thoughts, suicide plans, or suicide attempts; or among people with particular K6 or WHODAS scores (CBHSQ, 2015b).
3.4.7.12 Use of Break-Off Analysis Weights for 2020 Mental Illness Estimates among Adults
As described in Sections 2.3.4.2 and 3.3.2.1, the introduction of web-based data collection in Quarter 4 of 2020 increased item nonresponse due to respondents not completing the full survey (i.e., break-offs). Although statistical imputation was used to fill in missing values for many 2020 NSDUH measures, it was not used for all measures in the mental health section. Many of the mental health measures, especially those used in the SMI and AMI prediction models (e.g., K6 and WHODAS scores described in Sections 3.4.7.4 and 3.4.7.5, respectively), were zero imputed (e.g., missing data being treated as equivalent to responses of “none of the time” or “no difficulty”) because mental health measures have historically low missingness rates on the NSDUH.
For Quarter 4 of 2020, the bulk of missing data in usable interviews (Section 2.3.1) among adults were due to break-offs later in the survey. Treating break-offs as equivalent to other missing data (i.e., responses of “don’t know” or “refused”) in analyses will not bias estimates when the probability of a break-off does not depend on the characteristics of respondents who broke off. For data from the 2020 NSDUH interview that occurred during or after the mental health section for adults, however, it was more likely that breaking off was related to the characteristics of respondents who broke off. To reduce the potential bias that would arise from handling missing data due to break-offs the same way that other missing data were handled in analyses, break-off analysis weights were created for 2020 (Section 2.3.4.2). These break-off analysis weights were used to produce published 2020 estimates of AMI, SMI, and other estimates among adults that included AMI or SMI (e.g., use of mental health services in the past year among adults with AMI or SMI).
3.4.8 Major Depressive Episode (Depression)
Two sections related to MDE were included in the 2020 questionnaire, an adult depression and an adolescent depression section. These sections were originally derived from DSM-IV (APA, 1994) criteria for MDE. Consistent with the more recent criteria in the DSM-5 (APA, 2013), NSDUH does not exclude MDEs occurring exclusively in the context of bereavement.
Questions on depression permit estimates to be calculated for the occurrence of MDE in the population and receipt of treatment for MDE. Separate sections were administered to adults aged 18 or older and youths aged 12 to 17. The adult questions were adapted from the depression section of the National Comorbidity Survey Replication (NCS-R), and the questions for youths were adapted from the depression section of the National Comorbidity Survey Replication Adolescent Supplement (NCS-A).86 To make the sections developmentally appropriate for youths, there are minor wording differences in a few questions between the adult and youth sections. Revisions to the questions in both sections were made primarily to reduce their length and to modify the NCS questions, which were interviewer-administered, to the ACASI format used in NSDUH. In addition, some revisions, based on cognitive testing, were made to improve comprehension. Furthermore, even though titles similar to those used in the NCS were used for the NSDUH sections, the results of these items may not be directly comparable. Potential differences in results are mainly due to differing modes of administration in each survey (ACASI in NSDUH vs. computer-assisted personal interviewing [CAPI] in the NCS), revisions to wording necessary to maintain the logical processes of the ACASI environment, and possible context effects resulting from deleting questions not explicitly pertinent to severe depression.
According to DSM-5, people are classified as having had an MDE87 in their lifetime if they had at least five or more of nine symptoms nearly every day (except where noted) in the same 2-week period, where at least one of the symptoms is a depressed mood or loss of interest or pleasure in daily activities (APA, 2013). These symptoms are as follows: (1) depressed mood most of the day; (2) markedly diminished interest or pleasure in all or almost all activities most of the day; (3) significant weight loss when not sick or dieting, or weight gain when not pregnant or growing, or decrease or increase in appetite; (4) insomnia or hypersomnia; (5) psychomotor agitation or retardation at a level observable by others; (6) fatigue or loss of energy; (7) feelings of worthlessness or excessive or inappropriate guilt; (8) diminished ability to think or concentrate or indecisiveness; and (9) recurrent thoughts of death or suicidality (i.e., recurrent suicidal ideation without a specific plan, making a specific plan, or making an attempt). Unlike the other symptoms listed previously, recurrent thoughts of death or suicidality did not need to have occurred nearly every day.
Respondents who have had an MDE in their lifetime are asked if, during the past 12 months, they had a period of depression lasting 2 weeks or longer while also having some of the other symptoms mentioned. Respondents reporting experiences consistent with their having had an MDE in the past year are asked questions from the SDS to measure the level of functional impairment in major life activities reported to be caused by the MDE in the past 12 months (Leon et al., 1997). Responses to the SDS questions have not been used as predictors of SMI in NSDUH after 2008; for more information, see Section 3.4.7.
NSDUH measures the nine symptoms associated with MDE as defined in DSM-5 with the following questions. The questions shown are taken from the adult depression section of the 2020 NSDUH questionnaire. A few of the questions in the youth section were modified slightly to use wording more appropriate for youths aged 12 to 17. However, no exclusions were made for MDE caused by medication, alcohol, illicit drugs, or any medical illness.
- Depressed mood most of the day
The following questions refer to the worst or most recent period of time when the respondent experienced any or all of the following: sadness, discouragement, or lack of interest in most things.
During that [worst/most recent] period of time …
- … did you feel sad, empty, or depressed most of the day nearly every day?
- … did you feel discouraged about how things were going in your life most of the day nearly every day?
- Markedly diminished interest or pleasure in all or almost all activities most of the day
- … did you lose interest in almost all things like work and hobbies and things you like to do for fun?
- … did you lose the ability to take pleasure in having good things happen to you, like winning something or being praised or complimented?
- Weight
In answering the next questions, think about the [worst/most recent] period of time.
- Did you have a much smaller appetite than usual nearly every day during that time?
- Did you have a much larger appetite than usual nearly every day?
- Did you gain weight without trying to during that [worst/most recent] period of time?
- … because you were growing?
- … because you were pregnant?
- How many pounds did you gain?
- Did you lose weight without trying to?
- … because you were sick or on a diet?
- How many pounds did you lose?
- Insomnia or hypersomnia
- Did you have a lot more trouble than usual falling asleep, staying asleep, or waking too early nearly every night during that [worst/most recent] period of time?
- During that [worst/most recent] period of time, did you sleep a lot more than usual nearly every night?
- Psychomotor agitation or retardation
- Did you talk or move more slowly than is normal for you nearly every day?
- Did anyone else notice that you were talking or moving slowly?
- Were you so restless or jittery nearly every day that you paced up and down or couldn’t sit still?
- Did anyone else notice that you were restless?
- Fatigue or loss of energy
- During that [worst/most recent] period of time, did you feel tired or low in energy nearly every day, even when you had not been working very hard?
- Feelings of worthlessness
- Did you feel that you were not as good as other people nearly every day?
- Did you feel totally worthless nearly every day?
- Diminished ability to think or concentrate or indecisiveness
- During that [worst/most recent] time period, did your thoughts come much more slowly than usual or seem confused nearly every day?
- Did you have a lot more trouble concentrating than usual nearly every day?
- Were you unable to make decisions about things you ordinarily have no trouble deciding about?
- Recurrent thoughts of death or recurrent suicidal ideation
- Did you often think about death, either your own, someone else’s, or death in general?
- During that period, did you ever think it would be better if you were dead?
- Did you think about committing suicide?
Respondents who had missing data for whether they had an MDE in the past 12 months were excluded from the analyses to produce published estimates for the 2020 NSDUH. See Section 3.3.2 for a discussion of the potential bias in estimates because of missing data.
NSDUH also collects data on impairment using the SDS, which is a measure of impairment due to mental health issues in four major life activities or role domains. These four domains are defined separately for adults aged 18 or older and youths aged 12 to 17 to reflect the different roles associated with the two age groups. Each section consists of four questions, and each item uses an 11-point scale ranging from 0 (no interference for adults and no problems for adolescents) to 10 (very severe interference for adults and very severe problems for adolescents). The impairment score is defined as the single highest severity level of role impairment across the four SDS role domains. Ratings greater than or equal to 7 on the scale were considered severe impairment.
In addition to past year MDE, NSDUH shows estimates for past year MDE with severe impairment. Estimates for severe impairment are calculated separately for youths and adults because the four domains are slightly different for the two groups. Respondents who had missing data for impairment were excluded from the analyses to produce published estimates for MDE with severe impairment in the 2020 NSDUH. See Section 3.3.2 for a discussion of the potential bias in estimates because of missing data.
Because variables for lifetime and past year MDE among adults in 2020 were not imputed, the break-off analysis weights described in Section 2.3.4.2 were used to produce 2020 estimates for adults who had an MDE or any MDE with severe impairment in the past year. For youths aged 12 to 17, the main person-level analysis weight was used to produce estimates of MDE and MDE with severe impairment in the past year because the number of break-offs among youths was minimal for 2020.
3.4.8.1 Adult Depression Section: Functional Impairment
The questions pertaining to the four domains of functional impairment for adults aged 18 or older are listed below. The scale is shown below for the first domain but applies to all four domains.
- ASDSHOME
- Think about the time in the past 12 months when these problems with your mood were most severe.
- Using the 0 to 10 scale shown below, where 0 means no interference and 10 means very severe interference, select the number that describes how much these problems interfered with your ability to do each of the following activities during that period. You can use any number between 0 and 10 to answer.
- How much did your [depression symptoms] interfere with your ability to do home management tasks, like cleaning, shopping, and working around the house, apartment, or yard?
- ASDSWORK
- During that time in the past 12 months when your [depression symptoms] were most severe, how much did this interfere with your ability to work?
- ASDSREL
- How much did your [depression symptoms] interfere with your ability to form and maintain close relationships with other people during that period of time?
- ASDSSOC
- How much did [depression symptoms] interfere with your ability to have a social life during that period of time?
3.4.8.2 Youth Depression Section: Functional Impairment
The questions pertaining to the four domains of functional impairment for adolescents aged 12 to 17 are listed below. The scale is also shown below for the first domain but applies to all four domains.
- YSDSHOME
- Think about the time in the past 12 months when these problems with your mood were the worst.
- Using the 0 to 10 scale shown below, where 0 means no problems and 10 means very severe problems, select the number that describes how much your [depression symptoms] caused problems with your ability to do each of the following activities during that time. You can use any number between 0 and 10 to answer.
- How much did your [depression symptoms] cause problems with your chores at home?
- YSDSWORK
- During that time in the past 12 months when your [depression symptoms] were worst, how much did this cause problems with your ability to do well at school or work?
- YSDSREL
- How much did your [depression symptoms] cause problems with your ability to get along with your family during that time?
- YSDSSOC
- How much did your [depression symptoms] cause problems with your ability to have a social life during that time?
3.4.8.3 Adjustment of MDE Data for Context Effects
Since 2004, the NSDUH questions used to determine MDE have remained unchanged for both adults and youths. In the 2008 questionnaire, however, changes were made in other mental health items preceding the MDE questions (K6, suicide, and impairment) for adults. Questions also were retained in 2009 for the WHODAS impairment scale, and the questions for the SDS impairment scale were deleted; see Sections 3.4.6 and 3.4.7 of this report for further details about these questionnaire changes. The 2008 questionnaire changes affected the reporting on MDE questions among adults. Thus, adult MDE estimates for 2008 through 201988 cannot be directly compared with previously published unadjusted NSDUH adult MDE estimates based on data prior to 2008 or with unadjusted data from the 2008B half sample described in Section 3.4.7 of this report. See Sections B.4.4 and B.4.7 of the 2008 NSDUH’s national findings report (OAS, 2009b) for a further discussion of this comparability issue. In addition, estimates of adult MDE in 2008 included in the 2009 mental health findings report (CBHSQ, 2010) were based on only half of the sample (see Section 3.4.6 in this report).
To address the break in comparability of the adult MDE data beginning in 2008 and to estimate adult MDE based on the full sample of adults from 2008, adjusted versions of the lifetime and past year MDE variables for adults were created retroactively for 2005 to 2008. These variables were adjusted to make MDE estimates from the SDS half sample in 2008 and from all adult respondents for 2005 to 2007 comparable with the MDE estimates based on data from the half sample who received the WHODAS in 2008 and from all adult respondents in later years. The adjusted data from 2005 to 2008 were used in conjunction with unadjusted data from later years to estimate trends in adult MDE over the period from 2005 to 2019 and to include estimates for 2020.
Specifically, a weighted logistic regression was fit for the NSDUH data from 2005 to 2009 with past year MDE as the binary dependent variable. Independent variables in this model controlled for the questionnaire differences between NSDUHs from 2005 to 2007 and NSDUHs from 2008 and 2009, as well as for the context effects associated with the SDS half sample in 2008. This model was used to compute predicted probabilities of past year MDE for each respondent. The predicted probabilities, which can have any value between 0 and 1, then were dichotomized such that each respondent was specified as having or not having MDE in the past year. Adjusted lifetime MDE estimates were similarly constructed, with the additional condition that respondents reporting past year MDE were assumed to have lifetime MDE. Details about the adjustment of the adult MDE data for 2005 to 2008 can be found in a report describing these procedures (CBHSQ, 2012a).
In addition, changes in the YMHSU section questions in 2009 preceding the questions about adolescent depression could have affected adolescents’ responses to the adolescent depression questions and estimates of adolescent MDE. As discussed in Section 3.4.6 in this report, however, these changes in 2009 did not appear to affect the estimates of adolescent MDE. Therefore, data on trends in past year MDE from 2004 to 2009 did not require adjustment for adolescents aged 12 to 17.
3.4.9 Perceived Recovery
Four questions for adult respondents aged 18 or older were added to the end of the consumption of alcohol section for the 2018 NSDUH. These questions were moved to the emerging issues section that was added to the 2020 questionnaire and followed the consumption of alcohol section. Respondents first were asked whether they thought they ever have had a problem with their own drug or alcohol use. If adult respondents answered “yes,” they were asked whether they considered themselves to be in recovery or to have recovered from their own problem with drug or alcohol use. These first two questions on recovery from a substance use problem were followed by a set of two similar questions asking adult respondents if they have ever had a problem with their own mental health, and if so, if they considered themselves to be in recovery or to have recovered from their own mental health problem.
These perceived recovery estimates were included in tables and reports for the 2020 NSDUH. Estimates of perceived recovery were reported in the 2020 detailed tables among (1) adults who reported ever having a substance use problem or mental health issue and (2) all adults, regardless of whether they perceived themselves ever to have had a problem. To generate estimates among the total adult population, adults who reported not having a problem were classified as not being in recovery or having recovered from a problem. Respondents were excluded from analyses if they had unknown information for whether they ever had a substance use problem or mental health issue. Respondents also were excluded from analyses if they had unknown information for whether they perceived themselves to be in recovery or to have recovered from their respective problem (e.g., if respondents reported ever having had a substance use problem but did not know or refused to report whether they perceived themselves to be in recovery or to have recovered from their substance use problem). For a discussion on how procedures for handling missing data may bias estimates, see Section 3.3.2.
Consistent with the discussion in Section 3.3.4, data users also are reminded of a limitation: These estimates are based on self-reports of whether adult respondents thought they ever had a problem with their substance use or mental health and (if so) whether they perceived themselves to have recovered or to be in recovery from these problems. Specifically, these estimates reflect adults’ perceptions but not necessarily the clinical assessments of medical or mental health professionals. In addition, data on adults’ perceptions of whether they had a problem with their substance use or mental health and whether they perceived themselves to have recovered or to be in recovery from these problems were not edited relative to data in other sections of the interview for substance use, SUDs, substance use treatment, mental health issues, or the receipt of mental health services (see Section 2.3.2). Therefore, some data users may consider these perceptions to be inconsistent with substance use and mental health data from earlier sections of the interview. Nevertheless, if these questions remain constant over time and the extent of inconsistencies between these perceptions and clinical assessments (if they had been done) also remains relatively constant, then data users can make reliable conclusions about changes in these estimates, despite the potential limitations of the data.
The emerging issues section occurred in the 2020 NSDUH interview after the mental health and adult depression sections. Also, the perceived recovery variables were not imputed for 2020. Therefore, 2020 estimates for perceived recovery were created using the break-off analysis weight described in Section 2.3.4.2.
3.4.10 Medication-Assisted Treatment
Questions were added to the 2019 NSDUH interview in the consumption of alcohol section to measure the receipt of medication-assisted treatment (MAT) for alcohol and for opioids (heroin or prescription pain relievers). These questions were moved to the emerging issues section that was added to the 2020 questionnaire and followed the consumption of alcohol section. MAT was defined as medication prescribed by a doctor or other health professional to help reduce or stop the use of alcohol or opioids. MAT questions in NSDUH asked about the receipt of any MAT for alcohol or opioids in the past 12 months, specific medications used, and the frequency of use of specific medications in the past 12 months.
The MAT questions were asked only of respondents who reported receiving substance use treatment in the past 12 months. Specifically, NSDUH respondents aged 12 or older who reported receiving any treatment in the past 12 months for problems related to their use of alcohol were asked whether a doctor or other health professional prescribed them medication in that period to help reduce or stop their use of alcohol. Questions on MAT for opioid misuse were asked if respondents aged 12 or older reported ever using heroin or ever misusing prescription pain relievers and reported receiving any treatment in the past year for their use of illicit drugs. These respondents were asked whether a doctor or other health professional prescribed them medication in the past 12 months to help reduce or stop their use of heroin, misuse of prescription pain relievers, or both. Respondents also were informed that MAT for opioid misuse differed from medications given to stop a drug overdose.
MAT estimates appear in tables and reports for the 2020 NSDUH. These estimates show the receipt of any MAT for alcohol, for opioids, and for either alcohol or opioids. The 2020 detailed tables also report estimates of MAT for alcohol use among people with an alcohol use disorder and estimates of MAT for opioid misuse among people with an opioid use disorder.
Because the MAT questions were asked only of respondents who reported receiving substance use treatment in the past 12 months, respondents who did not receive substance use treatment in their lifetime or in the past 12 months were classified as not having received MAT. Similarly, respondents whose edited substance use treatment data indicated they received treatment in the past 12 months only for their use of illicit drugs were classified as not having received MAT for their use of alcohol. Respondents who never used heroin or misused prescription pain relievers or whose edited substance use treatment data indicated they received treatment in the past 12 months only for their use of alcohol were classified as not having received MAT for their misuse of opioids.
Respondents could have missing data for their receipt of MAT for several reasons.
- It was unknown whether respondents received substance use treatment in their lifetime or the past 12 months.
- Respondents received substance use treatment in the past 12 months, but whether they received treatment for their use of alcohol, illicit drugs, or both was unknown.
- Respondents were not asked whether they received MAT for their use of alcohol because they reported receiving substance use treatment in the past 12 months only for their use of illicit drugs. However, other data in the substance use treatment section indicated they last received treatment or were currently receiving treatment for their use of alcohol.
- Respondents were not asked whether they received MAT for their misuse of opioids because it was unknown whether they ever used heroin or misused prescription pain relievers.
- Respondents who misused opioids in their lifetime were not asked whether they received MAT for their misuse of opioids because they reported receiving substance use treatment in the past 12 months only for their use of alcohol. However, other data in the substance use treatment section indicated they last received treatment or were currently receiving treatment for their misuse of opioids.
For the MAT estimates in the detailed tables, respondents with missing data for receipt of MAT were classified as though they had not received MAT (i.e., zero filled). This method also was used for handling missing data for other substance use treatment measures reported in the detailed tables (see Section 3.4.4). However, some of these respondents with missing data could have received MAT in the past 12 months. Therefore, the zero fill method will cause a negative bias in the estimates (see Section 3.3.2).
In addition, the question for whether respondents received treatment in the past 12 months for their use of alcohol, illicit drugs, or both allowed respondents to report receiving treatment for their use of alcohol without previously reporting lifetime use of alcohol. Consequently, a small number of respondents in 2019 (fewer than 10) reported receiving MAT for their use of alcohol, but they did not previously report lifetime alcohol use. These respondents were not counted as having received alcohol MAT. This pattern did not occur in the 2020 data.
Data also could be inconsistent for MAT for opioid misuse and whether respondents misused opioids. For example, respondents could report misusing prescription pain relievers in the past 12 months and not report lifetime heroin use. However, respondents were logically inferred not to have misused prescription pain relievers in the past 12 months if they reported only the misuse of any other prescription pain reliever in that period and reported OTC drugs were the only pain relievers they misused (see Section 3.4.1); these respondents were not misusers of prescription pain relievers in the past 12 months, but whether they misused prescription pain relievers in their lifetime was unknown. Respondents were not counted in the estimates as having received opioid MAT in the past 12 months if they reported MAT for opioid misuse, but their status as lifetime opioid misusers was unknown because of their reports of misuse of only OTC drugs as other pain relievers.
The emerging issues section occurred in the 2020 NSDUH interview after the mental health and adult depression sections. Also, the MAT variables were not imputed for 2020. Therefore, 2020 estimates for the receipt of MAT data were created using the break-off analysis weight described in Section 2.3.4.2.
3.4.11 Kratom Use
Kratom is an herbal extract from the leaves of the Mitragyna speciosa tree native to Southeast Asia. The leaves contain chemicals with mind-altering effects. Kratom can come in forms such as powders, pills, or leaves. It can produce effects similar to those produced by stimulants or opioids, depending on the dose. Kratom is currently not classified nationally as a controlled substance, but some states may prohibit its possession and sale (NIDA, 2019a; U.S. Drug Enforcement Administration, 2017).
Starting with the 2019 NSDUH, respondents aged 12 or older were asked whether they ever used kratom and if so, how long it had been since they last used it. In the 2019 CAI instrument, these questions were placed at the end of the consumption of alcohol section. These questions were moved to the emerging issues section that was added to the 2020 questionnaire and followed the consumption of alcohol section. Estimates of lifetime, past year, and past month use of kratom are presented in the 2020 detailed tables (CBHSQ, 2021e).
Kratom use measures were imputed starting with the 2020 NSDUH instead of using the zero fill method for unknown responses. The kratom measures shown in the 2020 tables and reports were imputed for both 2019 and 2020. Therefore, the 2019 estimates presented in tables and reports for the 2020 NSDUH may differ from those presented in the prior NSDUH tables and reports. See Section 2.3.3 for more information on the imputation process.
3.4.12 Vaping and Creation of Aggregate Measures for Tobacco Use or Nicotine Vaping
Questions were added to the emerging issues section of the 2020 NSDUH interview to measure vaping of any substance and vaping of nicotine or tobacco with e-cigarettes or other vaping devices. NSDUH multiyear trend data from 2019 showed that past month cigarette smoking has been declining among people in all age groups (CBHSQ, 2020e). However, if people are vaping nicotine instead of using cigarettes or other tobacco products, then they could be trading one form of nicotine addiction for another. Thus, the introduction of these vaping questions into the NSDUH questionnaire closed an important gap in the NSDUH data.
3.4.12.1 Vaping of Any Substance
All respondents in 2020 were asked whether they had ever, even once, vaped anything with an e-cigarette or other vaping device. The examples given for the possible devices used were vape pens, personal vaporizers, or mods. The examples given for substances that people could have vaped were nicotine or tobacco, marijuana, flavoring, or other substances. If respondents reported that they ever vaped anything with an e-cigarette or other vaping device, then they were asked how long it has been since they last vaped anything with an e-cigarette or other vaping device (i.e., within the past 30 days, more than 30 days ago but within the past 12 months, or more than 12 months ago).
Because of how the questions for any vaping were structured, however, one cannot determine the specific substances that people vaped. For example, respondents could have vaped only marijuana or only nicotine flavoring, but it is not possible to discern from the data which specific substances respondents vaped. Consequently, data for vaping of any substance are not presented in the detailed tables or the key substance use and mental health indicators report for the 2020 NSDUH (CBHSQ, 2021e, 2021h).
3.4.12.2 Vaping of Nicotine
Respondents who reported that they vaped anything in their lifetime also were asked whether they ever vaped nicotine or tobacco with an e-cigarette or other vaping device. As for questions about vaping of any substance, respondents who reported that they ever vaped nicotine or tobacco were asked how long it had been since they last vaped nicotine or tobacco. Questions for the last time respondents vaped nicotine or tobacco were tailored according to their reports of when they last vaped any substance. This tailoring of questions was designed to reduce the opportunity for respondents to provide answers for when they last vaped nicotine or tobacco that were inconsistent with their reports of when they last vaped any substance. The tailoring of recency questions for vaping of nicotine or tobacco was as follows:
- If respondents previously reported that they last vaped any substance more than 12 months ago, then they were not asked when they last vaped nicotine or tobacco. Logically, these respondents last vaped nicotine or tobacco more than 12 months ago.
- If respondents reported that they last vaped any substance more than 30 days ago but within the past 12 months, they could report that they last vaped nicotine or tobacco more than 30 days ago but within the past 12 months or more than 12 months ago. However, these respondents were not allowed to report that they last vaped nicotine or tobacco within the past 30 days.
- If respondents reported that they last vaped any substance within the past 30 days or they did not know or refused to report when they last vaped any substance, then they could report that they last vaped nicotine or tobacco within the past 30 days, more than 30 days ago but within the past 12 months, or more than 12 months ago. If respondents last vaped any substance in the past 30 days, for example, then they could have last vaped nicotine or tobacco in any of these periods.
Estimates for the vaping of nicotine or tobacco were presented in tables and reports for the 2020 NSDUH. Missing data in these new nicotine vaping measures were imputed.
3.4.12.3 Tobacco Product Use or Nicotine Vaping
The NSDUH definition of the use of tobacco products has included the use of cigarettes, cigars, smokeless tobacco, or pipe tobacco. Beginning in 2020, new aggregate measures were also created and presented in NSDUH tables and reports that include the use of tobacco products (as defined previously) or nicotine vaping. Respondents who used tobacco products or vaped nicotine in their lifetime were classified for this aggregate measure as having used tobacco products or having vaped nicotine. Measures for the use of tobacco products or nicotine vaping in the lifetime, past year, or past month periods were created according to the most recent time when respondents used tobacco products or vaped nicotine. Because the measures for the most recent use of tobacco products and most recent nicotine vaping were imputed, aggregate measures for the use of tobacco products or nicotine vaping had no missing data.
3.4.13 Synthetic Marijuana Use or Synthetic Stimulant Use
Synthetic cannabinoids and synthetic cathinones are human-made chemicals with properties similar to naturally occurring chemicals in plants. Synthetic cannabinoids are similar to chemicals found in the marijuana plant. For this reason, these drugs are sometimes called “synthetic marijuana” or “fake weed.” They can be contained in plant material that is later smoked. They are also sold as liquids to be vaporized (i.e., vaped) and inhaled in e-cigarettes and other devices (NIDA, 2020b). Synthetic cathinones are central nervous system stimulants that are chemically related to cathinone, a substance found in the khat plant. These substances can be marketed as “bath salts” or “flakka” (NIDA, 2020c).
Several synthetic cannabinoids and synthetic cathinones have been categorized as Schedule I controlled substances, meaning that they have no currently accepted medical use and have a high potential for abuse (U.S. Drug Enforcement Administration, 2020b). The 2020 NSDUH marks the first time that information was collected in the survey on the use of synthetic cannabinoids and synthetic cathinones.
3.4.13.1 Synthetic Marijuana Use
For simplicity, the 2020 NSDUH questionnaire asked respondents about their use of “synthetic marijuana” rather than asking specifically about synthetic cannabinoids. The questionnaire also included the slang terms “fake weed,” “K2,” and “Spice” for questions about synthetic marijuana. The 2020 NSDUH asked respondents aged 12 or older if they ever used synthetic marijuana or fake weed and if so, how long it had been since they last used it. Estimates of lifetime, past year, and past month use of synthetic marijuana (along with the terms fake weed, K2, or Spice) are presented in the 2020 detailed tables (CBHSQ, 2021e). The 2020 key substance use and mental health indicators report presented estimates only for the past year use of synthetic marijuana (CBHSQ, 2021h).
Missing data for the lifetime use of synthetic marijuana were statistically imputed (see Section 2.3.3). Data for the most recent use of synthetic marijuana were statistically imputed if respondents had missing data for lifetime use (and, therefore, also for the most recent use) and were statistically imputed to be lifetime users. Data for the most recent use of synthetic marijuana also were statistically imputed if respondents reported lifetime use, but they did not report when they last used it.
3.4.13.2 Synthetic Stimulant Use
For simplicity, the 2020 NSDUH questionnaire asked respondents about their use of “synthetic stimulants” rather than asking specifically about synthetic cathinones. The questionnaire also included the slang terms “bath salts” and “flakka” for questions about synthetic stimulants. The 2020 NSDUH asked respondents aged 12 or older if they ever used synthetic stimulants, also called “bath salts” or flakka, and if so, how long it had been since they last used them. Estimates of lifetime, past year, and past month use of synthetic stimulants (along with the terms “bath salts” and flakka) are presented in the 2020 detailed tables (CBHSQ, 2021e). The 2020 key substance use and mental health indicators report presented estimates only for the past year use of synthetic stimulants (CBHSQ, 2021h).
Missing data for the lifetime use of synthetic stimulants were statistically imputed (see Section 2.3.3). Data for the most recent use of synthetic stimulants were statistically imputed if respondents had missing data for lifetime use (and, therefore, also for the most recent use) and were statistically imputed to be lifetime users. Data for the most recent use of synthetic stimulants also were statistically imputed if respondents reported lifetime use, but they did not report when they last used it.
3.4.14 Central Nervous System Stimulant Misuse
Central nervous system (CNS) stimulants are a group of drugs that include cocaine, methamphetamine, and prescription stimulants. These drugs act in similar ways to stimulate the brain. They produce stimulant effects, such as increased alertness, wakefulness, or energy. They also can produce physical side effects of rapid or irregular heartbeat or increased blood pressure and body temperature (NIDA, 2018b, 2019b, 2021b).
Therefore, an aggregate measure for CNS stimulant misuse was created for the 2020 NSDUH. Because this aggregate measure includes the misuse of prescription stimulants in addition to the use of cocaine or methamphetamine, it was defined as CNS stimulant misuse.
CNS stimulant misuse data are available for the past year and past month. Because of potential measurement issues for the lifetime misuse of prescription drugs (see Section 3.4.1), estimates for lifetime CNS stimulant misuse were not presented in tables and reports for the 2020 NSDUH. Measures for CNS stimulant misuse in the past year or past month periods were created according to the most recent time when respondents used or misused these substances. Because the measures were imputed for cocaine use, methamphetamine use, and prescription stimulant misuse for the past year and past month, the aggregate measures for CNS stimulant misuse in those periods had no missing data for 2020. Section 3.4.3.5 also describes the creation of measures for CNS stimulant use disorder.
3.4.15 Use of Virtual (Telehealth) Services
In response to the COVID-19 pandemic, healthcare providers (including behavioral healthcare providers) turned to virtual (telehealth) services (i.e., delivery of healthcare services over the phone or Internet) as a means of delivering services while also limiting in-person contact that could spread the COVID-19 virus (U.S. Department of Health and Human Services, 2021a). Options for behavioral health care providers to be reimbursed for providing virtual (telehealth) services were expanded during the pandemic to include services provided over the phone using only audio (U.S. Department of Health and Human Services, 2021b).
Questions on the provision of virtual (telehealth) services were added to the 2020 NSDUH questionnaire in Quarter 4 for substance use treatment, medical care, and mental health care.89 For each type of service, respondents were asked if they received service “over the phone, by email, or through video calling.” See Appendix A for definitions for these measures.
For tables and reports for the 2020 NSDUH, estimates for the receipt of virtual (telehealth) services were presented using only Quarter 4 data. Estimates of virtual (telehealth) services for substance use treatment or youth mental health service utilization were created using the main analysis weight for Quarter 4, with no adjustment for respondents who did not complete the interview (i.e., break-offs). Estimates of virtual (telehealth) mental health services for adults used the break-off analysis weight from Quarter 4 that is described in Section 2.3.4.2.
3.4.16 Suicidal Thoughts and Behavior
The 2020 NSDUH included questions asking adults aged 18 or older and adolescents aged 12 to 17 whether they had serious thoughts of suicide, made a suicide plan, or attempted suicide in the past 12 months. Respondents who reported that they made a suicide attempt were asked if they received medical attention or stayed overnight in the hospital because of their suicide attempt.
Questions about suicidal thoughts and behavior among youths were added to the 2020 NSDUH questionnaire for Quarter 4. Follow-up questions also were added in Quarter 4 if adults or youths reported suicidal thoughts or behavior. These follow-up questions asked if the suicidal thoughts or behavior were because of the COVID-19 pandemic.
3.4.16.1 Suicidal Thoughts and Behavior among Adults
In the mental health section of the 2020 NSDUH questionnaire, adult respondents were asked about suicidal thoughts and behaviors in the past 12 months. In all quarters of 2020, respondents who reported that they tried to kill themselves in the past 12 months were asked whether they received medical attention from a doctor or other health professional for their suicide attempt. If respondents reported receiving medical attention, they were asked whether they stayed overnight or longer in a hospital for their suicide attempt.
Before Quarter 4 of 2020, only those adult respondents who reported that they had serious thoughts of suicide in the past 12 months were asked if they made a suicide plan or tried to kill themselves. Beginning in Quarter 4, all adults were asked if they made a suicide plan or attempted suicide regardless of what they reported for serious thoughts of suicide. This revised skip logic will be used in the 2021 NSDUH.
Few adult respondents in Quarter 4 (fewer than 15) did not report that they had serious thoughts of suicide but they made suicide plans or attempted suicide. For estimates of suicide plans and suicide attempts that were based on the full year of 2020 data from Quarters 1 and 4, the Quarter 4 data were adjusted so that any respondents in Quarter 4 who reported not having serious thoughts of suicide in the past 12 months were treated in the analyses as not making suicide plans or attempting suicide in that period. This handling of Quarter 4 data was consistent with how corresponding data were handled in Quarter 1 and in prior years when respondents reported that they did not have serious thoughts of suicide in the past 12 months.
This issue of the changed skip logic in Quarter 4 also applied to estimates for the receipt of medical attention because of suicide attempts and hospitalization because of suicide attempts that were based on the full year of 2020 data from Quarters 1 and 4. Respondents in Quarter 4 who were handled in the analyses as not attempting suicide because they reported that they did not have serious thoughts of suicide in the past 12 months were also handled as not receiving medical attention or not staying overnight in a hospital because of a suicide attempt. These analysis procedures enabled consistency in the way that Quarter 4 data were handled compared with analyses in prior years.
Questions about suicidal thoughts and behavior among adults occurred in the mental health section for adults. Also, the variables for suicidal thoughts and behavior among adults were not imputed for 2020. Therefore, 2020 estimates for suicidal thoughts and behavior among adults were created using the break-off analysis weight described in Section 2.3.4.2.
3.4.16.2 Suicidal Thoughts and Behavior among Adolescents
As noted previously, questions were added to the NSDUH interview in Quarter 4, 2020 that asked about adolescents’ suicidal thoughts and behaviors in the past 12 months. These questions were added to the youth mental health service utilization section. As for questions about suicidal thoughts and behavior among adults, the questions for adolescents aged 12 to 17 asked whether respondents seriously thought about trying to kill themselves, made plans to kill themselves, or tried to kill themselves in the past 12 months. Adolescent respondents who reported that they made a suicide attempt were asked if they received medical attention or stayed overnight in the hospital because of their suicide attempt. Consistent with the revisions to the skip logic in Quarter 4 for adults described in Section 3.4.16.1, all respondents aged 12 to 17 were asked if they made a suicide plan or attempted suicide regardless of what they reported for serious thoughts of suicide. Unlike the questions for adults, the questions about suicidal thoughts and behavior among adolescents included response choices for “I’m not sure” and “I Don’t want to answer,” in addition to standard response choices for “yes” and “no.” Adolescent respondents also could choose these response choices for “I’m not sure” and “I Don’t want to answer” instead of using function keys (as is the practice elsewhere in the interview) for answers of “don’t know” or “refused,” respectively.
Estimates for suicidal thoughts and behavior among adolescents were included in tables and reports for the 2020 NSDUH. Because these data were available only from Quarter 4, analyses used the Quarter 4 main analysis weight that is described in Section 2.3.4.2. In addition, tables and reports for 2020 include estimates for “I’m not sure,” and “I Don’t want to answer,” in addition to estimates for “yes” and “no.” Responses of “don’t know” were grouped with “I’m not sure,” and refusals were grouped with “I Don’t want to answer.”
The 2020 estimates for suicidal thoughts and behavior among adolescents from Quarter 4 were created using the main analysis weights, with no adjustment because of break-offs. As discussed in Section 2.3.4.2, investigations of the 2020 NSDUH data from Quarter 4 indicated that a smaller number of adolescents aged 12 to 17 broke off the interview before they reached the youth mental health service utilization section where the questions were located for suicidal thoughts and behavior among adolescents.
3.4.16.3 Suicidal Thoughts and Behavior Because of the COVID-19 Pandemic
Questions also were added in Quarter 4 of 2020 for adults and adolescents about suicidal thoughts and behavior because of the COVID-19 pandemic. If respondents in Quarter 4 reported that they seriously thought about trying to kill themselves, made plans to kill themselves, or tried to kill themselves in the past 12 months, they were asked follow-up questions for whether the particular suicidal thought or behavior was because of the COVID-19 pandemic. For example, if respondents reported that they seriously thought about trying to kill themselves in the past 12 months (for any reason), they were asked, “Was this because of the COVID-19 pandemic?” Estimates were presented in tables and reports for the 2020 NSDUH for whether people attributed their suicidal thoughts or behavior to the COVID-19 pandemic.
As noted previously, questions about suicidal thoughts and behavior among adults occurred in the mental health section for adults, including the questions about suicidal thoughts and behaviors because of the COVID-19 pandemic. These variables for suicidal thoughts and behavior among adults because of the COVID-19 pandemic were not imputed for 2020. Therefore, estimates from Quarter 4 of 2020 for suicidal thoughts and behavior among adults because of the COVID-19 pandemic were created using the break-off analysis weight described in Section 2.3.4.2.
Small numbers of adolescents in Quarter 4 reported suicidal thoughts or behavior for any reason. Therefore, estimates of suicidal thoughts and behavior among adolescents because of COVID-19 were suppressed because of low precision (see Section 3.2.2 and Table 3.2). For example, estimates for serious thoughts of suicide because of COVID-19 among all adolescents who had serious thoughts of suicide were suppressed because of low precision.
3.4.17 Perceived Effects of the COVID-19 Pandemic
Researchers have raised concerns that the COVID-19 pandemic could have negative effects on substance use and mental health outcomes (Czeisler et al., 2020; Hossain et al., 2020; Torales et al., 2020). People also could have experienced economic problems or difficulty accessing services for substance use treatment, mental health care, or medical care because of the pandemic. For example, in the first week of data collection (April 23 to May 5, 2020) for the Household Pulse Survey (Section 5.1.3), 38.8 percent of adults expected to have a loss in employment income in the next 4 weeks (U.S. Census Bureau, n.d.). The estimated percentage of adults from this survey who delayed or did not get care in the last 4 weeks ranged from 44.5 to 45.7 percent over the period from May 7 to July 21, 2020 (National Center for Health Statistics, n.d.-b).
Therefore, questions were added to the 2020 NSDUH questionnaire for Quarter 4 on the following topics related to the COVID-19 pandemic in the United States:
- how much the pandemic negatively affected respondents’ emotional or mental health since the beginning of the pandemic;
- how much the pandemic affected the amount of alcohol respondents drank (if they used alcohol in the past 12 months);
- how much the pandemic affected respondents’ use of drugs other than alcohol (if they used illicit drugs90 in the past 12 months);
- how often respondents had serious financial worries because of the pandemic;
- whether respondents were homeless, living on the street, in a vehicle, or in some type of makeshift housing at any time because of the pandemic;
- whether respondents experienced the following in their access to mental health treatment because of the pandemic:
- appointments moved from in-person to telehealth,
- delays or cancellations in appointments,
- delays in getting prescriptions, or
- the inability to access needed care, resulting in a moderate to severe impact on their health;
- whether respondents experienced specific issues in their access to substance use treatment because of the pandemic (same issues as those listed for the access to mental health treatment); and
- whether respondents experienced specific issues in their access to medical care because of the pandemic (same issues as those listed for the access to mental health treatment and access to substance use treatment).
Tables and reports for the 2020 NSDUH present estimates for these topics.
Questions about these topics related to the COVID-19 pandemic occurred in the 2020 NSDUH questionnaire after the mental health and adult depression sections. Also, the variables for perceived effects of the COVID-19 pandemic were not imputed for 2020. Therefore, estimates for perceived effects of the COVID-19 pandemic were created using the break-off analysis weight described in Section 2.3.4.2.
Table 3.1 – Demographic and Geographic Domains Shown in the First Findings Reports and Detailed Tables Using the Alternative Standard Error Estimation Method for Calculating Standard Errors of the Estimated Number of People (Totals), Quarters 1 and 4, 2020
| Main Effects1 |
Quarters 1 and 4 Combined
Two-Way Interactions2,3 |
Quarter 1
Two-Way Interactions3 |
Quarter 4
Two-Way Interactions3 |
| Age Group |
Age Group × Gender
(e.g., males aged 12 to 17)
Hispanic Origin × Age Group (12-17,
18-25, 26-34, 35 or older, and
collapsed categories from this list)
(e.g., Hispanics or Latinos aged 18
to 25)
Age Group × Geographic Region
(e.g., people aged 12 to 25 in the
Northeast)
Gender × Hispanic Origin
(e.g., not Hispanic or Latino
males)
Hispanic Origin × Race (White,
non-White others)
(e.g., not Hispanic or Latino
Whites) |
Age Group × Gender
(e.g., males aged 12 to 17)
Hispanic Origin × Age Group (12-17,
18-25, 26-34, 35- 49, 50 or older, and
collapsed categories from this list)
(e.g., Hispanics or Latinos aged 18
to 25)
Age Group × Geographic Region
(e.g., people aged 12 to 25 in the
Northeast)
Gender × Hispanic Origin
(e.g., not Hispanic or Latino males)
Hispanic Origin × Race (White, non-
White others)
(e.g., not Hispanic or Latino Whites) |
Age Group × Gender
(e.g., males aged 12 to 17)
Hispanic Origin × Age Group (12-17,
18-25, 26-34, 35 or older, and
collapsed categories from this list)
(e.g., Hispanics or Latinos aged 18
to 25)
Age Group × Geographic Region
(e.g., people aged 12 to 25 in the
Northeast)
Gender × Hispanic Origin
(e.g., not Hispanic or Latino males)
Hispanic Origin × Race
(e.g., not Hispanic or Latino
Whites) |
| 12-17 |
| 18-25 |
| 26-34 |
| 35-49 |
| 50-64 |
| 65 or Older |
| Collapsed Age Group Categories from Above4 |
| |
| Gender |
| Male |
| Female |
| |
| Hispanic Origin |
| Hispanic or Latino |
| Not Hispanic or Latino |
| |
| Race5 |
| White |
| Black or African American |
| Others |
| |
| Geographic Region |
| Northeast |
| Midwest |
| South |
| West |
| |
| Education (18 or Older)6 |
| Less than High School |
| High School Graduate |
| Some College/Associate’s Degree |
| College Graduate |
NOTE: The alternative standard error (SE) estimation method for the estimated number of people (totals), 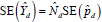 , is applied when the domain size estimates, , is applied when the domain size estimates, 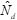 , are among those forced to match their respective U.S. Census Bureau or American Community Survey (ACS) population estimates through the weight calibration process. , are among those forced to match their respective U.S. Census Bureau or American Community Survey (ACS) population estimates through the weight calibration process.
NOTE: The alternative SE estimation method does not affect the SEs for the corresponding means and proportions. These latter SEs are calculated directly in SUDAAN (RTI International, 2013), whereas the alternative SE estimation method is computed outside of SUDAAN using the formula provided in the first note.
NOTE: This table shows only the domains and domain combinations used in the National Survey on Drug Use and Health’s (NSDUH’s) first finding reports and detailed tables. Other domains and domain combinations (omitted here) also use this alternative SE estimation method, but they are not included in these specific reports or tables. For example, methodological studies or special requests often include a wider variety of domains and survey years. This variation requires the SE method to be assessed for each individual analysis. For a detailed list of domains for NSDUH forced to match their respective U.S. Census Bureau or ACS population estimates through the weight calibration process, see the 2020 person-level sampling weight calibration report (Center for Behavioral Health Statistics and Quality [CBHSQ], forthcoming c).
NOTE: The domains using the alternative standard error estimation method for calculating the standard error of the estimated number of people (total) are the same for both the main analysis weight and the break-off analysis weight (Section 2.3.4 of this report for more details about these two weights). However, the domains in 2020 are slightly different from those used for 2019 and prior years. See Chapter 3 of the 2019 methodological summary and definitions report (CBHSQ, 2020c) for details about the 2019 domains.
1 The main effects are the same for Quarter 1, Quarter 4, and Quarters 1 and 4 combined.
2 The combined Quarters 1 and 4 two-way interactions are a combination of the two-way interactions of the individual quarters (i.e., the more restrictive of the Quarter 1 and Quarter 2 two-way interactions). No separate weight calibration was done for the combined Quarters 1 and 4 weights (both the main and break-off analysis weights); instead, the combined Quarter 1 and 4 weights were created by dividing the separate nonzero Quarter 1 and 4 analysis weights by 2. See Section 2.3.4 of this report for more details.
3 Unless otherwise noted, the domains for the two-way interactions are the same as the main effect domains (including the collapsed age categories). Two-way interactions involving age group include the main effect and collapsed age group categories. If age groups are listed in the two-way interaction columns, then only those age groups can be collapsed to form broader age categories.
4 Main effect age group categories shown in the table can be collapsed to form broader age group categories (e.g., 12 or older, 50 or older, 18 to 49, 26 to 49). Collapsed main effect age group categories and two-way interactions with other main effect demographic or geographic domains shown (e.g., males aged 50 or older) also use the alternative SE estimation method because the collapsed main effects will sum to the census totals for the category being defined. However, broader age groups that include only a subset of the main effect age groups (e.g., 12 to 20, 21 or older, 15 to 44), age groups finer than the main effect age groups (e.g., 12 to 13, 18 to 20), or two-way interactions of these types of collapsed age categories with other main effect domains (e.g., females aged 15 to 44) should not use the alternative SE estimation method.
5 Race is included as a main effect in this table for completeness; however, race groups presented here include all people within a given race category, regardless of whether they are Hispanic or not Hispanic. In contrast, all other groups presented in the detailed tables are indented under the “Non-Hispanic” ethnicity row heading. For example, the domain for Whites in the detailed tables is actually non-Hispanic Whites and is therefore a two-way interaction. Thus, any additional domains crossed with non-Hispanic Whites (e.g., Whites aged 18 to 25) represent three-way interactions not using the alternative SE estimation method.
6 For 2020, education was added as a main effect in the weighting process. Education categories are only defined for respondents aged 18 or older in NDSUH’s first finding reports and detailed tables. Thus, education is shown in the main effect column of this table because the 18 or older age group is considered the full population for education.
Source: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2020. |
|---|
Table 3.2 – Summary of 2020 NSDUH Suppression Rules
| Estimate |
Suppress if: |
Prevalence rate,  , with nominal sample size, n, and design effect, deff , with nominal sample size, n, and design effect, deff
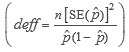 |
(1) The estimated prevalence rate,  , is < .00005 or > .99995, or , is < .00005 or > .99995, or
(2) 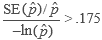 when when 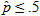 , or , or
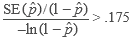 when when 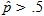 , or , or
(3) Effective n < 68, where 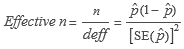 , or , or
(4) n < 100.
Note: The rounding portion of this suppression rule for prevalence rates will produce some estimates rounded at one decimal place to 0.0 or 100.0 percent but are not suppressed. |
Estimated number
(numerator of  ) ) |
The estimated prevalence rate,  , is suppressed. , is suppressed.
Note: In some instances when  is not suppressed, the estimated number may appear as a 0. This means the estimate is greater than 0 but less than 500 (estimated numbers are shown in thousands). is not suppressed, the estimated number may appear as a 0. This means the estimate is greater than 0 but less than 500 (estimated numbers are shown in thousands). |
Means not bounded between 0 and 1
(e.g., mean age at first use), 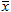 , with nominal sample size, n , with nominal sample size, n |
(1) 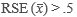 , or , or
(2) n < 10. |
deff = design effect; RSE = relative standard error; SE = standard error.
NOTE: Starting in 2020 for confidentiality protection, survey sample sizes greater than 100 were rounded to the nearest 10, and sample sizes less than 100 were not reported (i.e., are shown as “<100” in tables).
Source: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2020. |
Figure 3.1 Required Effective Sample in the 2020 NSDUH as a Function of the Proportion Estimated
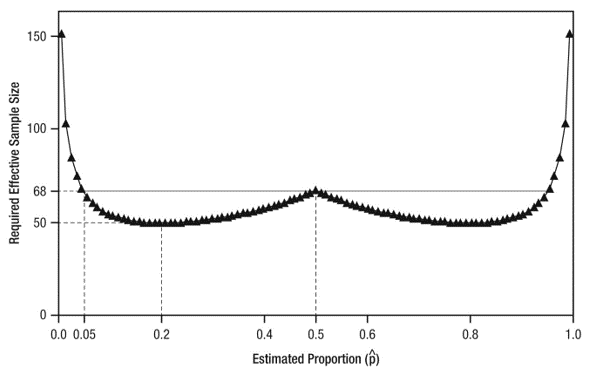 D
D
Table 3.3 – Final Screening Result Code: Weighted Percentages and Sample Sizes, 2020
Final Screening Result
Code |
Sample
Size 2020 |
Sample
Size 2020
Quarter 1 |
Sample
Size 2020
Quarter 4 |
Weighted
Percentage
2020 |
Weighted
Percentage
2020
Quarter 1 |
Weighted
Percentage
2020
Quarter 4 |
| TOTAL SAMPLE |
642,549 |
60,785 |
581,764 |
100.00 |
100.00 |
100.00 |
| Ineligible Cases |
106,346 |
7,958 |
98,388 |
15.66 |
13.29 |
16.46 |
| Eligible Cases |
536,203 |
52,827 |
483,376 |
84.34 |
86.71 |
83.54 |
| INELIGIBLES |
106,346 |
7,958 |
98,388 |
15.66 |
13.29 |
16.46 |
| 10 - Vacant |
4,920 |
4,382 |
538 |
11.71 |
52.92 |
0.60 |
| 13 - Not a Primary Residence |
2,220 |
1,802 |
418 |
5.40 |
23.78 |
0.44 |
| 18 - Not a Dwelling Unit |
671 |
578 |
93 |
1.58 |
7.19 |
0.07 |
| 22 - All Military Personnel |
228 |
86 |
142 |
0.35 |
1.07 |
0.16 |
| Other, Ineligible1 |
98,307 |
1,110 |
97,197 |
80.95 |
15.04 |
98.73 |
| ELIGIBLE CASES |
536,203 |
52,827 |
483,376 |
84.34 |
86.71 |
83.54 |
| Screening Complete |
90,937 |
35,304 |
55,633 |
25.71 |
67.76 |
11.13 |
| 30 - No One Selected |
46,803 |
18,053 |
28,750 |
13.04 |
33.90 |
5.80 |
| 31 - One Selected |
25,569 |
10,027 |
15,542 |
7.36 |
19.57 |
3.13 |
| 32 - Two Selected |
18,565 |
7,224 |
11,341 |
5.31 |
14.29 |
2.20 |
| Screening Not Complete |
445,266 |
17,523 |
427,743 |
74.29 |
32.24 |
88.87 |
| 11 - No One Home/No Contact Made |
43,721 |
3,605 |
40,116 |
6.82 |
6.52 |
6.92 |
| 12 - Respondent Unavailable/Web Nonrespondent |
384,864 |
727 |
384,137 |
60.72 |
1.58 |
81.23 |
| 14 - Physically or Mentally Incapable |
277 |
228 |
49 |
0.12 |
0.43 |
0.01 |
| 15 - Language Barrier - Hispanic |
137 |
117 |
20 |
0.07 |
0.27 |
0.00 |
| 16 - Language Barrier - Other |
348 |
333 |
15 |
0.19 |
0.73 |
0.00 |
| 17 - Refusal |
12,813 |
9,547 |
3,266 |
5.14 |
18.07 |
0.65 |
| 21 - Other, Access Denied2 |
2,828 |
2,720 |
108 |
1.12 |
4.23 |
0.04 |
| 24 - Other, Eligible |
98 |
90 |
8 |
0.05 |
0.16 |
0.01 |
| 27 - Segment Not Accessible |
0 |
0 |
0 |
0.00 |
0.00 |
0.00 |
| 33 - Screener Not Returned |
28 |
6 |
22 |
0.01 |
0.02 |
0.00 |
| 39 - Fraudulent Case |
152 |
150 |
2 |
0.06 |
0.23 |
0.00 |
| 44 - Electronic Screening Problem |
0 |
0 |
0 |
0.00 |
0.00 |
0.00 |
1 Examples of “Other, Ineligible” cases are those in which all residents lived in the dwelling unit for less than half of the calendar quarter and dwelling units listed in error.
2 “Other, Access Denied” includes all dwelling units to which the field interviewer was denied access, including locked or guarded buildings, gated communities, and other controlled access situations.
Source: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2020. |
Table 3.4 – Final Interview Code: Weighted Percentages and Sample Sizes, Combined Quarters 1 and 4, 2020
| Final Interview Code |
12 or Older
Sample Size
Quarters 1 and 4
Combined |
12 or Older
Weighted
Percentage
Quarters 1 and 4
Combined |
12 to 17
Sample Size
Quarters 1 and 4
Combined |
12 to 17
Weighted
Percentage
Quarters 1 and 4
Combined |
18 or Older
Sample Size
Quarters 1 and 4
Combined |
18 or Older
Weighted
Percentage
Quarters 1 and 4
Combined |
| TOTAL |
62,515 |
100.00 |
14,313 |
100.0 |
48,202 |
100.00 |
| 70 - Interview Complete |
36,284 |
60.41 |
6,337 |
36.78 |
29,947 |
62.79 |
| 71 - No One at Dwelling Unit/Web Nonrespondent |
14,713 |
23.29 |
6,007 |
54.20 |
8,706 |
20.18 |
| 72 - Respondent Unavailable |
2,006 |
2.42 |
395 |
1.97 |
1,611 |
2.47 |
| 73 - Break-Off |
640 |
1.80 |
5 |
0.08 |
635 |
1.97 |
| 74 - Physically/ Mentally Incapable |
415 |
0.64 |
86 |
0.38 |
329 |
0.67 |
| 75 - Language Barrier - Hispanic |
76 |
0.07 |
10 |
0.03 |
66 |
0.08 |
| 76 - Language Barrier - Other |
130 |
0.29 |
0 |
0.00 |
130 |
0.32 |
| 77 - Refusal |
6,732 |
9.85 |
403 |
1.81 |
6,329 |
10.66 |
| 78 - Parental Refusal |
1,014 |
0.39 |
1,014 |
4.32 |
0 |
0.00 |
| 91 - Fraudulent Case |
15 |
0.02 |
15 |
0.21 |
0 |
0.00 |
| Other1 |
490 |
0.81 |
41 |
0.22 |
449 |
0.87 |
NOTE: Some eligible and selected people at the household screening stage were later determined to be ineligible based on information obtained at the interviewing stage. These ineligible people are not included in the table.
1 “Other” includes eligible person moved, data not received from field, too dangerous to interview, access to building denied, computer problem, and interviewed wrong household member.
Source: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2020. |
Table 3.5 – Final Interview Code: Weighted Percentages and Sample Sizes, Quarter 1, 2020
| Final Interview Code |
12 or Older
Sample Size
Quarter 1 |
12 or Older
Weighted
Percentage
Quarter 1 |
12 to 17
Sample Size
Quarter 1 |
12 to 17
Weighted
Percentage
Quarter 1 |
18 or Older
Sample Size
Quarter 1 |
18 or Older
Weighted
Percentage
Quarter 1 |
| TOTAL |
24,304 |
100.00 |
5,692 |
100.00 |
18,612 |
100.00 |
| 70 - Interview Complete |
15,628 |
63.24 |
3,936 |
70.53 |
11,692 |
62.51 |
| 71 - No One at Dwelling Unit/Web Nonrespondent |
925 |
3.37 |
146 |
2.21 |
779 |
3.48 |
| 72 - Respondent Unavailable |
1,359 |
5.74 |
292 |
5.46 |
1,067 |
5.77 |
| 73 - Break-Off |
10 |
0.06 |
1 |
0.10 |
9 |
0.06 |
| 74 - Physically/ Mentally Incapable |
354 |
2.10 |
71 |
1.15 |
283 |
2.20 |
| 75 - Language Barrier - Hispanic |
69 |
0.25 |
9 |
0.11 |
60 |
0.26 |
| 76 - Language Barrier - Other |
123 |
1.11 |
0 |
0.00 |
123 |
1.23 |
| 77 - Refusal |
4,732 |
21.98 |
331 |
5.31 |
4,401 |
23.67 |
| 78 - Parental Refusal |
868 |
1.31 |
868 |
14.32 |
0 |
0.00 |
| 91 - Fraudulent Case |
2 |
0.00 |
2 |
0.05 |
0 |
0.00 |
| Other1 |
234 |
0.83 |
36 |
0.76 |
198 |
0.84 |
NOTE: Some eligible and selected people at the household screening stage were later determined to be ineligible based on information obtained at the interviewing stage. These ineligible people are not included in the table.
1 “Other” includes eligible person moved, data not received from field, too dangerous to interview, access to building denied, computer problem, and interviewed wrong household member.
Source: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2020. |
Table 3.6 – Final Interview Code: Weighted Percentages and Sample Sizes, Quarter 4, 2020
| Final Interview Code |
12 or Older
Sample Size
Quarter 4 |
12 or Older
Weighted
Percentage
Quarter 4 |
12 to 17
Sample Size
Quarter 4 |
12 to 17
Weighted
Percentage
Quarter 4 |
18 or Older
Sample Size
Quarter 4 |
18 or Older
Weighted
Percentage
Quarter 4 |
| TOTAL |
38,211 |
100.00 |
8,621 |
100.00 |
29,590 |
100.00 |
| 70 - Interview Complete |
20,656 |
59.48 |
2,401 |
25.60 |
18,255 |
62.88 |
| 71 - No One at Dwelling Unit/Web Nonrespondent |
13,788 |
29.86 |
5,861 |
71.42 |
7,927 |
25.69 |
| 72 - Respondent Unavailable |
647 |
1.33 |
103 |
0.81 |
544 |
1.38 |
| 73 - Break-Off |
630 |
2.38 |
4 |
0.08 |
626 |
2.61 |
| 74 - Physically/ Mentally Incapable |
61 |
0.16 |
15 |
0.13 |
46 |
0.16 |
| 75 - Language Barrier - Hispanic |
7 |
0.02 |
1 |
0.00 |
6 |
0.02 |
| 76 - Language Barrier - Other |
7 |
0.02 |
0 |
0.00 |
7 |
0.03 |
| 77 - Refusal |
2,000 |
5.84 |
72 |
0.65 |
1,928 |
6.37 |
| 78 - Parental Refusal |
146 |
0.09 |
146 |
1.01 |
0 |
0.00 |
| 91 - Fraudulent Case |
13 |
0.02 |
13 |
0.27 |
0 |
0.00 |
| Other1 |
256 |
0.80 |
5 |
0.04 |
251 |
0.88 |
NOTE: Some eligible and selected people at the household screening stage were later determined to be ineligible based on information obtained at the interviewing stage. These ineligible people are not included in the table.
1 “Other” includes eligible person moved, data not received from field, too dangerous to interview, access to building denied, computer problem, and interviewed wrong household member.
Source: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2020. |
Table 3.7 – Interview Response Rates and Sample Sizes; by Demographic Characteristics, Quarters 1 and 4, 2020
Demographic
Characteristic |
Selected
People
Combined
Quarters 1
and 4 |
Selected
People
Quarter 1 |
Selected
People
Quarter 4 |
Completed
Interviews
Combined
Quarters 1
and 4 |
Completed
Interviews
Quarter 1 |
Completed
Interviews
Quarter 4 |
Weighted
Response
Rate
Combined
Quarters 1
and 4 |
Weighted
Response
Rate
Quarter 1 |
Weighted
Response
Rate
Quarter 4 |
| TOTAL |
62,515 |
24,304 |
38,211 |
36,284 |
15,628 |
20,656 |
60.41% |
63.24% |
59.48% |
| AGE GROUP |
|
|
|
|
|
|
|
|
|
| 12-17 |
14,313 |
5,692 |
8,621 |
6,337 |
3,936 |
2,401 |
36.78% |
70.53% |
25.60% |
| 18-25 |
15,223 |
5,793 |
9,430 |
8,978 |
3,680 |
5,298 |
57.29% |
63.43% |
55.26% |
| 26 or Older |
32,979 |
12,819 |
20,160 |
20,969 |
8,012 |
12,957 |
63.65% |
62.36% |
64.07% |
| GENDER |
|
|
|
|
|
|
|
|
|
| Male |
30,350 |
12,078 |
18,272 |
16,782 |
7,584 |
9,198 |
57.60% |
61.46% |
56.36% |
| Female |
32,165 |
12,226 |
19,939 |
19,502 |
8,044 |
11,458 |
63.09% |
64.89% |
62.48% |
| RACE/ETHNICITY |
|
|
|
|
|
|
|
|
|
| Hispanic |
9,946 |
4,376 |
5,570 |
5,393 |
2,801 |
2,592 |
54.32% |
63.59% |
51.20% |
| White, Not Hispanic |
40,097 |
15,000 |
25,097 |
23,658 |
9,514 |
14,144 |
62.56% |
63.36% |
62.29% |
| Black, Not Hispanic |
5,785 |
2,543 |
3,242 |
3,386 |
1,767 |
1,619 |
58.90% |
65.51% |
56.65% |
| All Other Races, Not Hispanic |
6,687 |
2,385 |
4,302 |
3,847 |
1,546 |
2,301 |
59.28% |
58.20% |
59.61% |
| REGION |
|
|
|
|
|
|
|
|
|
| Northeast |
13,076 |
4,702 |
8,374 |
7,191 |
2,751 |
4,440 |
57.62% |
57.68% |
57.61% |
| Midwest |
15,469 |
5,892 |
9,577 |
9,103 |
3,775 |
5,328 |
62.57% |
63.19% |
62.36% |
| South |
18,385 |
7,727 |
10,658 |
10,992 |
5,225 |
5,767 |
61.65% |
66.12% |
60.15% |
| West |
15,585 |
5,983 |
9,602 |
8,998 |
3,877 |
5,121 |
58.57% |
62.53% |
57.27% |
| COUNTY TYPE |
|
|
|
|
|
|
|
|
|
| Large Metropolitan |
28,359 |
11,047 |
17,312 |
16,205 |
6,853 |
9,352 |
59.90% |
60.85% |
59.58% |
| Small Metropolitan |
23,310 |
8,099 |
15,211 |
13,465 |
5,262 |
8,203 |
60.50% |
64.93% |
59.20% |
| Nonmetropolitan |
10,846 |
5,158 |
5,688 |
6,614 |
3,513 |
3,101 |
62.26% |
68.64% |
59.74% |
NOTE: Estimates are based on demographic information obtained from screener data and are not consistent with estimates on demographic characteristics presented in the 2020 detailed tables (CBHSQ, 2021e).
Source: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2020. |
Table 3.8 – Comparison of SUD Criteria for DSM-5 and DSM-IV and Availability for the CVS and Non-CVS Respondents, 2020
| Criterion1 |
Used in
DSM-5 SUD
Classification |
Used in
DSM-IV SUD
Classification |
Available for CVS
Respondents2 |
Available for Non-CVS
Respondents2 |
| 1: Substance is often taken in larger amounts, longer than intended |
Yes |
Yes |
Yes, in DSM-5 SUD section |
Yes, in DSM-IV SUD section |
| 2: Unsuccessful efforts to cut down/control use |
Yes |
Yes |
Yes, in DSM-5 SUD section |
Yes, in DSM-IV SUD section |
| 3: A great deal of time is spent obtaining, using, recovering |
Yes |
Yes |
Yes, in DSM-5 SUD section |
Yes, in DSM-IV SUD section |
| 4: Craving/strong urge to use |
Yes |
No |
Yes, in DSM-5 SUD section2 |
Yes, but not in DSM-IV SUD section; added to emerging issues section later in the 2020 questionnaire |
| 5: Recurrent use resulting in failure to fulfill major role obligations at work/school/home |
Yes |
Yes |
Yes, in DSM-5 SUD section |
Yes, in DSM-IV SUD section |
| 6: Continued use despite social problems |
Yes |
Yes |
Yes, in DSM-5 SUD section |
Yes, in DSM-IV SUD section |
| 7: Important social/occupational/ recreational activities given up or reduced because of use |
Yes |
Yes |
Yes, in DSM-5 SUD section |
Yes, in DSM-IV SUD section |
| 8: Recurrent use in physically hazardous situations |
Yes |
Yes |
Yes, in DSM-5 SUD section2 |
Yes, in DSM-IV SUD section |
| 9: Continued use despite physical, psychological problems |
Yes |
Yes |
Yes, in DSM-5 SUD section |
Yes, in DSM-IV SUD section |
| 10: Increased amount of substance is needed to achieve same effect |
Yes |
Yes |
Yes, in DSM-5 SUD section |
Yes, in DSM-IV SUD section |
11a: Withdrawal symptoms
(symptoms and requirements differ by substance)
(Not applicable for hallucinogens/inhalants) |
Yes |
Yes |
Yes, in DSM-5 SUD section |
Yes, but not in DSM-IV SUD section for marijuana and Rx tranquilizers; added to emerging issues section later in the 2020 questionnaire for these two substances Yes, in DSM-IV SUD section for all other substances |
11b: The same or related substance is taken to avoid withdrawal symptoms3
(Not applicable for hallucinogens/inhalants) |
Yes |
No3 |
Yes, in DSM-5 SUD section |
No |
| X: Legal problems |
No |
Yes |
Yes, at the end of the DSM-5 SUD section |
Yes, in DSM-IV SUD section |
CVS = Clinical Validation Study; DSM = Diagnostic and Statistical Manual of Mental Disorders; Rx = prescription; SUD = substance use disorder.
1 The criterion wording is based on the 2020 NSDUH CVS questions; wordings based on the non-CVS questions may differ slightly.
2 CVS respondents in Quarter 1 received revised SUD questions based on the DSM-5 criteria. Non-CVS respondents in Quarter 1 received the DSM-IV SUD questions from 2019 plus additional questions in the emerging issues section later in the interview. After Quarter 1, all respondents received the non-CVS questions.
3 Criterion 11b was not included in the DSM-IV SUD NSDUH questions for any substance, despite it being part of the DSM-IV SUD criteria for all substances except hallucinogens and inhalants.
Source: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2020. |
Table 3.9 – Final SMI Prediction Models in the 2008 to 2012 Mental Health Surveillance Studies
| Variable |
Beta |
Beta SE |
T Statistic |
P Value |
DF |
Wald P
Value1 |
| WHODAS Sample (2008A-2012) |
| Intercept |
−5.9726640 |
0.3201 |
−18.6586 |
0.0000 |
|
|
| Alt PY K6 |
0.0873416 |
0.0248 |
3.5247 |
0.0009 |
1 |
0.0009 |
| Alt WHODAS |
0.3385193 |
0.0349 |
9.7034 |
0.0000 |
1 |
0.0000 |
| PY Suicidal Thoughts |
1.9552664 |
0.2164 |
9.0342 |
0.0000 |
1 |
0.0000 |
| PY MDE |
1.1267330 |
0.2196 |
5.1308 |
0.0000 |
1 |
0.0000 |
| Age1830 |
0.1059137 |
0.0244 |
4.3380 |
0.0001 |
1 |
0.0001 |
| WHODAS and SDS Samples (2008-2012)2 |
| Intercept |
−5.7736246 |
0.3479 |
−16.5960 |
0.0000 |
|
|
| Alt PY K6 |
0.1772067 |
0.0190 |
9.3251 |
0.0000 |
1 |
0.0000 |
| PY Suicidal Thoughts |
1.8392433 |
0.1941 |
9.4781 |
0.0000 |
1 |
0.0000 |
| PY MDE |
1.6428623 |
0.2119 |
7.7528 |
0.0000 |
1 |
0.0000 |
| Age1830 |
0.1231266 |
0.0259 |
4.7482 |
0.0000 |
1 |
0.0000 |
Age1830 = recoded age variable; Alt = alternative; DF = degrees of freedom; K6 = Kessler-6, a six-item psychological distress scale; MDE = major depressive episode; PY = past year; SDS = Sheehan Disability Scale; SE = standard error; SMI = serious mental illness; WHODAS = eight-item World Health Organization Disability Assessment Schedule.
NOTE: Alt PY K6: past year K6 score of < 8 recoded as 0; past year K6 score of 8 to 24 recoded as 1 to 17.
NOTE: Alt WHODAS: WHODAS item score of < 2 recoded as 0; WHODAS item score of 2 to 3 recoded as 1, then summed for a score ranging from 0 to 8.
NOTE: PY suicidal thoughts: coded as 1 if respondent had serious thoughts of suicide in the past year; coded as 0 otherwise.
NOTE: PY MDE: coded as 1 if the criteria for past year MDE were met; coded as 0 otherwise.
NOTE: Age1830: coded as age minus 18 if aged 18 to 30; coded as 12 otherwise.
1 The Wald p value is obtained from the overall model fitting.
2 The model is fit over the WHODAS and SDS samples in 2008-2012 but is used only to produce predictions for the 2008 SDS sample.
Source: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2008-2012. |
5. Other Sources of Data
The National Survey on Drug Use and Health (NSDUH) provides estimates of substance use and mental health issues for the civilian, noninstitutionalized population aged 12 years or older in the United States. Surveys and data systems other than NSDUH also produce findings for substance use and mental health indicators. Reviewing information from multiple national data sources, such as those included in this chapter, can provide data users with a more complete understanding of substance use and mental health issues among the U.S. population, including subpopulations not covered by NSDUH. Care should be taken, though, in direct comparisons of estimates from NSDUH and other surveys because methodological differences may lead to differences in the estimates across surveys.
The 2020 survey year was different for NSDUH and other national surveys that primarily collect data via in-person interviews. The Interagency Council on Statistical Policy compiled a summary report of data collection changes made by federal agencies in response to the coronavirus disease 2019 (COVID-19) pandemic (Interagency Council on Statistical Policy, 2021). In March 2020, in-person data collection for national surveys was suspended due to the COVID-19 pandemic. Plans to resume data collection differed appreciably among national surveys and included (1) stopping indefinitely and waiting to resume data collection when in-person contact was once again feasible; (2) stopping temporarily to redesign some aspect of the data collection, then resuming with a different design; and (3) continuing to collect data with an evolving design that excluded in-person data collection. For NSDUH, the major change in 2020 data collection was adding a web survey component and resuming in-person data collection where feasible in Quarter 4 (i.e., October to December 2020). Using the context of the array of 2020 data collection changes to national in-person surveys as a backdrop, this chapter describes other data sources that provide information on substance use and mental health indicators, including treatment for substance use problems or the receipt of mental health services.
Other reports also provide details comparing estimates from NSDUH and other data sources. These reports include comparisons on the following topics: substance use estimates for adolescents (Center for Behavioral Health Statistics and Quality [CBHSQ], 2012b); substance use estimates among adult male arrestees (Lattimore et al., 2014); estimates of health conditions and health care utilization (Pemberton et al., 2013); and data about utilization of substance use treatment (Batts et al., 2014). For mental health indicators, further information about the data sources described in this chapter and other data systems can be found in a report comparing NSDUH mental health data and methods with those from other data sources (Hedden et al., 2012) and a report on suicidal thoughts and behaviors (Miller et al., 2015). These and other CBHSQ reports can be found at https://www.samhsa.gov/data/.
It is important for data users to understand the methodological differences between data sources and the impact that these differences could have on estimates of substance use and mental health issues, even when other data sources also cover segments of the civilian, noninstitutionalized population of the United States. Methodological differences that can affect data include, but are not limited to, the populations covered (or not included), sample size and design, timing of data collection, mode of data collection, instruments used, operational definitions, and estimation methods. Such differences could be further compounded in 2020 as surveys made methodological changes, such as changing or adding modes to collect data, during the COVID-19 pandemic.
A survey’s purpose also can affect the breadth and depth of substance use and mental health issues being measured and the context in which substance use and mental health questions appear to survey respondents. Consequently, even when data users compare estimates between NSDUH and other surveys that cover the same population (or segments of the same population) as NSDUH, differences in substance use and mental health estimates across surveys may not mean that one set of estimates is more accurate than the other. Given the possible methodological differences among data sources, similarities in what these sources tell data users about substance use and mental health issues in the United States may be more worth emphasizing than the differences (CBHSQ, 2012b; Harrison, 2001).
When NSDUH and other data sources cover notably different populations (e.g., the civilian population for NSDUH vs. active-duty military personnel for other studies), readers also are reminded that demographic differences across populations can partially explain differences in substance use and mental health outcomes (in addition to influences of the population environment on these outcomes). Nevertheless, data from populations other than the civilian, noninstitutionalized population can indicate special needs of members of these other populations.
5.1 National Surveys Collecting Substance Use or Mental Health Data in the Civilian, Noninstitutionalized Population
5.1.1 Behavioral Risk Factor Surveillance System (BRFSS)
The Behavioral Risk Factor Surveillance System (BRFSS)—a state-based system of health surveys—collects information on health risk behaviors, preventive health practices, and health care access primarily related to chronic disease and injury. The BRFSS surveys are cross-sectional telephone surveys conducted by state health departments with technical and methodological assistance from the Centers for Disease Control and Prevention (CDC). Every year, states conduct monthly telephone surveys of adults (aged 18 or older) in households using random-digit-dialing methods; unlike NSDUH, BRFSS excludes people living in group quarters (e.g., dormitories). More than 400,000 adults from all 50 states, the District of Columbia, Guam, and Puerto Rico are interviewed each year (CDC, 2019). Since 2011, the BRFSS sample has included households with only cellular telephones in addition to those that were covered by landline telephones.
The BRFSS questionnaire has three parts: (1) a core questionnaire, (2) optional modules, and (3) state-added questions. The core questionnaire consists of a standard set of questions asked by all states every year. Thus, the core questionnaire allows for the creation of a common set of estimates across states, the District of Columbia, and participating U.S. territories. The core questionnaire includes questions on demographic characteristics, alcohol use, and tobacco use; the core component also includes rotating core questions that are included in even- and odd-numbered years. Questions about lifetime depression have been included in the core questionnaire since 2011. Optional modules consist of questions on specific topics that states can elect to include. Although the modules are optional, CDC standards require that states use them without modification. Optional modules have addressed topics such as (but not limited to) cancer survivorship, marijuana use, mental health (e.g., anxiety, depression, or psychological distress), and sexual orientation and gender identity. However, the number of states administering optional modules can vary from year to year.108 States also may include and analyze state-added questions at their own expense, but these questions are not part of the official BRFSS questionnaire.
Because BRFSS is a telephone survey, sampled adults could still be contacted during the COVID-19 pandemic because in-person contact was not required for data collection. Beginning in April 2020, for example, the Florida BRFSS added eight questions about sources of COVID-19 information and prevention behaviors (Gunderson et al., 2021). To reduce COVID-19 transmission and infections among telephone interviewers, telephone survey data collection could have needed to transition from on-site call centers to virtual call centers that would allow interviewers to work remotely (Lerner et al., 2021).
An important consideration for comparing NSDUH and BRFSS estimates is that the surveys use different statistics for central tendency that can yield different results, even if measures are comparable (e.g., for binge alcohol use109). National estimates in NSDUH represent weighted percentages of the entire civilian, noninstitutionalized population aged 12 or older or percentages of all individuals in a given subgroup (e.g., adults aged 18 or older); NSDUH respondents in states with larger populations contribute more heavily to national estimates than respondents in states with smaller populations. In contrast, because BRFSS data are collected at the state (or territory) level, national estimates for all 50 states and the District of Columbia from the online analysis tool or in publications that cite BRFSS data typically are presented as median percentages, independent of the size of a state’s population.110 In 2017, for example, NSDUH estimated that 26.4 percent of adults were binge alcohol users in the past month (CBHSQ, 2018a). BRFSS indicated a median prevalence of 17.4 percent for binge drinking among adults for the 50 states and the District of Columbia. Because of the different statistics for central tendency, therefore, the higher NSDUH estimate is not necessarily an indication of greater accuracy.
Differences in measures also can affect NSDUH and BRFSS estimates. For example, current cigarette use is defined in NSDUH as any cigarette use in the 30 days prior to the interview. BRFSS defines adults as current cigarette users if they smoked 100 or more cigarettes in their lifetime and reported they now smoke cigarettes every day or some days. In 2019, NSDUH estimated that 18.1 percent of adults were current cigarette smokers (CBHSQ, 2020a). BRFSS indicated a median prevalence of 16.0 percent for current cigarette smoking among adults for the 50 states and the District of Columbia.
Other methodological differences can also affect comparability between NSDUH and BRFSS estimates. First, NSDUH utilizes audio computer-assisted self-interviewing (ACASI) for administration of sensitive questions, whereas BRFSS uses computer-assisted telephone interviewing (CATI); self-administration (including ACASI for in-person data collection) can increase respondent privacy for reporting of sensitive behaviors and therefore may yield higher prevalence estimates than interviewer-administered modes such as CATI (Kreuter et al., 2008; Lind et al., 2013; Tourangeau & Smith, 1996; Turner et al., 1998). Second, because NSDUH has been conducted solely as an in-person survey (until 2020) and BRFSS is conducted as a telephone survey, response rates also have been higher in NSDUH than BRFSS, which could result in differential nonresponse bias patterns for common estimates in the two surveys.
For further details, see the BRFSS website at https://www.cdc.gov/brfss/.
5.1.2 Monitoring the Future (MTF)
Monitoring the Future (MTF) is an ongoing study of substance use trends and related attitudes among America’s secondary school students, college students, and adult high school graduates through age 60. MTF provides information on the use of alcohol, illicit drugs, and tobacco. The study is conducted annually by the Institute for Social Research at the University of Michigan through grants awarded by the National Institute on Drug Abuse (NIDA), which is part of the National Institutes of Health (NIH). MTF and NSDUH are the federal government’s largest and primary tools for tracking youth substance use. MTF is composed of three substudies: (1) an annual survey of high school seniors that was initiated in 1975, (2) ongoing panel studies of representative samples from each graduating class (i.e., 12th graders) that have been conducted (principally) by mail since 1976 (see below for changes in the 2018 and 2019 surveys), and (3) annual surveys of 8th and 10th graders that were initiated in 1991. Beginning in 2020, students in the 8th, 10th, and 12th grades completed a self-administered questionnaire on electronic tablets during a regular class period instead of using the traditional paper-and-pencil questionnaire.111 Because of the COVID-19 pandemic, in-school data collection for MTF was halted on March 15, 2020. Consequently, the sample size for the in-school data collection in 2020 was about one fourth of the typical sample size (i.e., typically more than 40,000 students in nearly 400 public and private secondary schools). Analyses indicated the smaller 2020 sample did not differ from nationally representative results from previous years in terms of demographic characteristics and substance use prevalence estimates that had been stable in recent years (Miech et al., 2021).
In addition, approximately 2,400 respondents who participated in the survey of 12th graders are followed longitudinally.112 In 2018 and 2019, the longitudinal follow-up component included a split sample among adults aged 19 to 30, in which a random half of the sample received the standard mail survey and the other half received a web survey. MTF moved to the web survey for all adults in this age group beginning in 2020, with paper-and-pencil questionnaires available by request (Schulenberg et al., 2019). The COVID-19 pandemic would not be expected to affect data collection for the longitudinal follow-up component.
Selected substance use measures common to NSDUH and MTF are shown in Tables 5.1 to 5.3 at the end of this chapter.113 For most substances in MTF, use in the lifetime, past 12 months, and past 30 days is determined from responses to questions about the number of occasions (if any) respondents used a substance in the period of interest, with use on zero occasions indicating nonuse in that period. MTF questions about cigarette use allow respondents to skip the question about cigarette use in the past 30 days if they report never smoking cigarettes. To allow the survey to cover multiple topics and reduce burden, MTF respondents are randomly allocated to receive different forms of the questionnaire. Consequently, sample sizes can vary for different substance use measures. MTF reports percentages but not estimated numbers of individuals because the study does not create separate analysis weights for substance use estimates from only a subsample of respondents (CBHSQ, 2012b).
Comparisons between the MTF estimates for 8th, 10th, and 12th graders and NSDUH estimates for adolescents aged 12 to 17 generally have shown NSDUH substance use prevalence levels to be lower than MTF estimates (see Tables 5.1 to 5.3 and Figures 5.1 to 5.4 at the end of this chapter).114 The lower estimates in NSDUH may be due to more underreporting in the household setting as compared with the MTF school setting and some overreporting in the school settings (Fowler & Stringfellow, 2001). Asking MTF respondents to report the number of times they have used a substance also could yield higher estimates if the frequency-of-use format suggests to adolescents that some substance use may be normative (CBHSQ, 2012b; Harrison, 2001), but it also could result in overreporting if nonusers answer the frequency questions incorrectly. In comparison, NSDUH uses “yes/no” questions for substances other than prescription drugs that allow respondents to skip remaining questions about that substance if they do not report lifetime use. Prior to the 2020 surveys, however, NSDUH and MTF have generally shown parallel trends in the prevalence of substance use for youths.
The population of inference for the MTF school-based data collection is adolescents who were in the 8th, 10th, and 12th grades; therefore, the MTF does not survey dropouts. The MTF also does not include students who were absent from school on the day of the survey, although they are part of the population of inference. NSDUH has shown that dropouts and adolescents who frequently were absent from school have higher rates of illicit drug use (CBHSQ, 2012b; Gfroerer et al., 1997b). Data from the Current Population Survey (CPS) indicate that the percentages of adolescents and young adults who were not currently enrolled in school and had not graduated from high school (i.e., school dropouts) increase as they get older.115 Depending on the effects of the exclusion of dropouts and frequent absentees, data from MTF may not generalize to the population of adolescents as a whole, especially for older adolescents.
For further details, see the MTF website at http://www.monitoringthefuture.org/  .
.
5.1.3 Household Pulse Survey
The U.S. Census Bureau, in collaboration with multiple federal agencies,116 designed the Household Pulse Survey to be deployed quickly and efficiently to collect data on the social and economic effects of COVID-19 on American households. Data have been disseminated in near real time to inform federal and state response and recovery planning.
The Household Pulse Survey asks questions about how COVID-19 has affected childcare, education, employment, food security, health, housing, social security benefits, household spending, consumer spending associated with stimulus payments, intention to receive a COVID-19 vaccination, and transportation. Data for the Household Pulse Survey were collected via a 20-minute online questionnaire. Phase 1 ran from April 23 to July 21, 2020; Phase 2 ran from August 19 to October 26, 2020; and Phase 3 ran from October 28, 2020, through March 29, 2021. Phase 3.1 of the survey began on April 14, 2021, and was expected to run through July 5, 2021.
As suggested by the National Center for Health Statistics (NCHS), the survey included modified versions of the two-item Patient Health Questionnaire (PHQ-2) to assess symptoms of depression and the two-item Generalized Anxiety Disorder (GAD-2) scale. The survey asked about symptoms over the last 7 days rather than the past 14 days.117 Occurrences of symptoms of depressive disorder from late April 2020 to May 10, 2021, ranged from 21.8 percent for the period from April 28 to May 10, 2021, to 30.2 percent from December 9 to 21, 2020. Occurrences of symptoms of anxiety disorder over this period ranged from 26.4 percent for the period from April 28 to May 10, 2021, to 37.2 percent from November 11 to 23, 2020 (NCHS, n.d.-a). However, estimates in data tables are not aggregated by phase or across phases.
Household Pulse Survey data indicate changes in mental health indicators among adults since April 2020. Without a baseline using pre-COVID-19 pandemic data that were collected using the same methods, however, conclusions from these data need to be made with caution for how the COVID-19 pandemic has affected the mental health of adults in the United States. Although corresponding mental health estimates from the 2019 National Health Interview Survey (NHIS) have been suggested as benchmarks, comparisons between the Household Pulse Survey and the 2019 NHIS could be misleading because the surveys used different modes of data collection (in person for the 2019 NHIS vs. web only for the Household Pulse Survey). In addition, response rates have been considerably lower in the Household Pulse Survey. In the first 3 weeks of data collection from April to May 2020, response rates ranged from 1.3 to 3.8 percent. The highest response rate was from August 19 to September 14, 2020 (10.3 percent). In comparison, the 2019 NHIS had a household response rate of 61.1 percent and a final sample adult response rate of 59.1 percent (NCHS, 2020).
Given the quick turnaround and the single data collection mode of the Household Pulse Survey, comparison of mental health estimates with those from NSDUH also should be made with caution. First, the web-only mode of data collection for the Household Pulse Survey excluded members of the target sample population because of the timing of data collection or because they could not access the Internet to complete the survey. Second, NSDUH and the Household Pulse Survey used different measures (e.g., two questions in the Household Pulse Survey to estimate depression symptoms vs. a more extensive set of questions in NSDUH to estimate major depressive episode in the lifetime and past 12 months). Third, the Household Pulse Survey questionnaire does not include complicated follow-up questions or complex skip patterns. Differences in questionnaire structure could affect how respondents answered questions and, therefore, the final estimates for each survey.
The U.S. Census Bureau categorizes the Household Pulse Survey as an experimental data product and notes the following on its website: “Census Bureau experimental data may not meet all of our quality standards. Because of this, we clearly identify experimental data products and include methodology and supporting research with their release” (U.S. Census Bureau, 2021).
For further details, see https://www.census.gov/programs-surveys/household-pulse-survey.html and https://www.census.gov/data/experimental-data-products/household-pulse-survey.html.
5.1.4 National Comorbidity Survey (NCS) Series (NCS, NCS-R, and NCS-A)
Studies in the National Comorbidity Survey (NCS) series have been designed to measure the prevalence, risk factors, and consequences of psychiatric morbidity and comorbidity among the general population. These studies also collected information on the use of alcohol, illicit drugs, and tobacco and the occurrence of substance use disorders (SUDs). These surveys were the precursors for the expansion of mental health questions in NSDUH.
If data users wish to compare estimates from NSDUH and the NCS series, important issues for them to consider are discussed below. Additional issues may apply to specific studies in the NCS series.
- Estimates from the NCS series of studies are several decades old. Therefore, this section does not compare estimates from NSDUH and the NCS series.
- Diagnostic criteria for mental disorders and SUDs have changed over time.
- Modes of administration, instrumentation, and estimation methods differ between NSDUH and studies in the NCS series.
The following studies were included in the NCS series:
- the original NCS, which was conducted between 1990 and 1992;
- the National Comorbidity Survey Replication (NCS-R), which was conducted from 2001 to 2003; and
- the National Comorbidity Survey Replication Adolescent Supplement (NCS-A), which was conducted from 2001 to 2004.
These studies were conducted by the University of Michigan’s Survey Research Center and were sponsored by the National Institute of Mental Health (NIMH), NIDA, and the William T. Grant Foundation. The NCS-R and NCS-A also received supplemental support from the Substance Abuse and Mental Health Services Administration (SAMHSA), the Robert Wood Johnson Foundation, and the John W. Alden Trust.
The NCS was an interviewer-administered household survey of individuals in the continental United States (i.e., excluding Alaska and Hawaii) that collected data from 8,098 respondents aged 15 to 54 using paper-and-pencil interviewing. The NCS used a modified version of the Composite International Diagnostic Interview (the University of Michigan [UM]-CIDI) to estimate the prevalence of mental disorders according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 3rd revised edition (DSM-III-R) (American Psychiatric Association [APA], 1987).
The NCS-R was conducted with a newly recruited, nationally representative, multistage, clustered-area probability sample of 9,282 U.S. respondents aged 18 or older (Kessler et al., 2004a). As in the NCS, the sample for the NCS-R excluded Alaska and Hawaii. Unlike the NCS, interviews for the NCS-R were conducted using computer-assisted personal interviewing (CAPI). The NCS-R also used criteria from the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (APA, 1994), rather than DSM-III-R criteria, for measuring substance use and mental disorders. Specifically, the NCS-R used a modified version of the World Mental Health Version of the Composite International Diagnostic Interview (WMH-CIDI) (Kessler & Üstün, 2004) to generate diagnoses according to the definitions and criteria of DSM-IV.
The NCS-A was designed to estimate the lifetime and current prevalence, age of onset, course, and comorbidity of DSM-IV disorders among adolescents in the United States; to identify risk and protective factors for the onset and persistence of these disorders; to describe patterns and correlates of service use for these disorders; and to lay the groundwork for subsequent follow-up studies that can be used to identify early expressions of adult mental disorders. The NCS-A consisted of a sample of adolescents aged 13 to 17. The sample included 904 adolescents from households that participated in the NCS-R and 9,244 respondents from a nationally representative sample of 320 public and private secondary schools (Kessler et al., 2009). Similar to the NCS and NCS-R, the sample for the NCS-A excluded Alaska and Hawaii. All adolescents were interviewed in their homes using CAPI. The NCS-A interview was similar in many ways to the adult NCS-R interview schedule. However, the NCS-A differed from the NCS-R in terms of diagnoses and questioning about risk factors and social consequences. The NCS-A also included self-administered questionnaires for parents.
For further details, see the NCS website at https://www.hcp.med.harvard.edu/ncs/  . Also, see the 2019 NSDUH methodological summary and definitions report (CBHSQ, 2020c) for additional details about these surveys.
. Also, see the 2019 NSDUH methodological summary and definitions report (CBHSQ, 2020c) for additional details about these surveys.
5.1.5 Uniform Reporting System (URS)
The NCS data mentioned previously that were collected between 1990 and 1992 have been used by the Uniform Reporting System (URS) of the Center for Mental Health Services (CMHS) to produce state-level estimates of serious mental illness (SMI) (Kessler et al., 2003a, 2003b, 2006). Using data from the NCS and the Baltimore site of the Epidemiologic Catchment Area (ECA) research project from 1980 to 1985, methods were developed to estimate SMI (Kessler et al., 1996, 1998, 2001). The definition of SMI was operationalized as respondents having met the following criteria: (1) presence of a “severe” and persistent mental illness as defined by the National Advisory Mental Health Council of NIMH (National Advisory Mental Health Council, 1993) or (2) respondents with another past 12-month DSM-III-R mental disorder (excluding “V” codes in the DSM,118 SUDs, and developmental disorders) and a planned suicide, attempted suicide, lack of a productive role, serious role impairment, or serious interpersonal impairment (Kessler et al., 1996, 2001). Impairment was assessed using questions that were included in the NCS and the ECA for other purposes (Kessler et al., 2001; Narrow et al., 2002).
Specifically, the URS selected a method for estimating state-level SMI prevalence that used the combined NCS data and data from the Baltimore site of the ECA by applying a model that controlled for demographic and geographic characteristics and corresponding census data (Kessler et al., 1998, 2004b). CMHS (1999) announced this methodology in the Federal Register as its final procedure for estimating the number of adults with SMI within each state. Through the URS, CMHS has continued to provide state and national estimates of the prevalence of SMI among the civilian population aged 18 years or older that fix the national SMI prevalence at 5.4 percent. Estimates of SMI by state are updated annually by applying updated population characteristics when new population data become available through the U.S. Census Bureau. Notably, this estimation method assumes that the prevalence of SMI in the adult population within the modeled demographic and geographic categories is homogeneous across states and does not change over time.
Several important differences between NSDUH and the URS that could affect estimates of mental illness warrant discussion. Most importantly, the URS assumes a national prevalence of SMI of 5.4 percent based on research conducted in the mid-1980s and the assumption that estimates for Baltimore hold true for the rest of the nation. In contrast, the 2020 NSDUH estimates are based on a statistical model developed using clinical interview data from separate subsamples of NSDUH respondents that were collected in 2008 to 2012, in combination with data from NSDUH interviews for all adults that were conducted in 2020. The difference between the research periods on which the SMI estimates are based is a key distinction between NSDUH and the URS. In particular, SMI estimates using the pooled NCS and ECA data used the Diagnostic and Statistical Manual of Mental Disorders, 3rd edition (APA, 1980), and DSM-III-R diagnostic criteria. NSDUH interview data were based on DSM-IV criteria.
For more details, see https://www.samhsa.gov/data/data-we-collect/urs-uniform-reporting-system.
5.1.6 National Health and Nutrition Examination Survey (NHANES)
The National Health and Nutrition Examination Survey (NHANES) has assessed the health and nutritional status of children and adults in the United States since the 1960s through the use of both survey and physical examination components. It is sponsored by NCHS and began as a series of periodic surveys in which several years of data were combined into a single data release. Since 1999, it has been a continuous survey, with interview data collected each year for approximately 5,000 individuals of all ages. The target population for NHANES is the civilian, noninstitutionalized population from birth onward. In early 2020, NHANES began to release aggregated public use data for 2017-2018; 2 years of data are combined to protect respondent confidentiality. Data are released to public use files on a flow basis.119
NHANES data collection was suspended in mid-March 2020 because of the COVID-19 pandemic, with the plan to resume in-person data collection when feasible in 2021. Therefore, this section describes procedures for the period before the COVID-19 pandemic and planned procedures for when data collection will resume.120
When the survey is in the field, NHANES interviews are conducted in respondents’ homes. NHANES includes two components: a household interview component that is administered through CAPI and a mobile examination center (MEC) component that collects physical health measurements and data on sensitive topics through ACASI; MECs travel to locations throughout the United States.
The NHANES household interview component includes a family questionnaire that collects household- and family-level information and a sample person questionnaire that collects individual-level information on the selected participants.121 In the household interview component, NHANES participants who were aged 16 or older answer for themselves; a proxy respondent provides information for participants who were younger than 16 or who could not answer themselves. The CAPI interviews were conducted in English or Spanish. The ACASI instrument in the MEC component is available in English, Spanish, and the following Asian languages: Chinese (traditional or simplified Mandarin or Cantonese), Korean, and Vietnamese.122 In the 2017 and 2018 NHANES, 16,211 individuals were selected, 9,254 completed the household interview, and 8,704 were examined.
Questions about cigarette smoking were administered to adult respondents in the household interview component using CAPI. The NHANES MEC interview also includes questions on alcohol, illicit drug, and tobacco use. The sample person questionnaire for NHANES (administered through CAPI) also asks respondents123 whether they used or took medication in the past 30 days “for which a prescription is needed.”124 Thus, NSDUH and NHANES differ in ways that affect the comparability of prescription drug data: (1) the different reference periods (past 12 months for NSDUH and past 30 days for NHANES), (2) the types of questions (e.g., NHANES respondents being asked to show containers of prescription drugs, which could encourage respondents to report use of medications for which they had legitimate prescriptions and to underreport misuse of medications without a prescription of their own), (3) mode of administration (ACASI in NSDUH and CAPI in NHANES), (4) whether proxy respondents (in NHANES) or the respondents themselves (in NSDUH) answered for sample members aged 12 to 15, (5) sample sizes (68,073 respondents aged 12 or older in the 2015 NSDUH vs. 7,201 respondents in this age range for the 2013-2014 NHANES125), and (6) when the data were collected.
Sources of nonresponse and coverage bias also differ for the two surveys. For example, NHANES respondents have to travel to an MEC to respond to substance use items other than tobacco use for adults. This feature may exclude homebound respondents or affect the participation of respondents with limited access to transportation. In addition, the principal focus of NSDUH on substance use and mental health issues versus the presentation of substance use questions in NHANES in the context of a broader array of health issues also could affect the comparability of estimates.
For further details, see the NHANES website at https://www.cdc.gov/nchs/nhanes/index.htm.
5.1.7 National Health Interview Survey (NHIS)
The National Health Interview Survey (NHIS) is a continuous, nationally representative sample survey sponsored by the NCHS. The survey provides national estimates of the health status, access to care and insurance, health service utilization, and health behaviors of the civilian, noninstitutionalized population, including cigarette smoking and alcohol use among adults aged 18 or older. NHIS data have been collected since 1957. There have been four main components of the survey in recent years: the Household Composition section, which collects basic demographic and relationship information for all individuals in the household; the Family Core, which collects information about all family members, typically from a respondent (the “household respondent”) who is of legal majority age in the state;126 the Sample Adult Core (including questions about cigarette smoking and alcohol use), which collects information from one adult aged 18 or older in each family; and the Sample Child Core, which collects information on youths under age 18 from a knowledgeable family member, usually a parent, in households with a child.
Sample sizes before 2020 were relatively large. For example, the 2017 NHIS public use file had data for 32,617 households containing 78,132 individuals. Sample sizes for the Sample Adult Core and Sample Child Core were 26,742 and 8,845, respectively. The household response rate was 66.5 percent. Final response rates were 65.7 percent for the Family Core, 53.0 percent for the Sample Adult Core, and 60.2 percent for the Sample Child Core (NCHS, 2018a).
The 2020 NHIS underwent multiple changes in response to the COVID-19 pandemic. Standard in-person household data collection procedures were in place until March 19, 2020, with interviews being conducted through CAPI. On March 19, NHIS temporarily became a telephone survey, with sampled addresses being matched to telephone numbers using commercial lists and additional searches where possible. Consequently, the household response rate declined from 60 percent in Quarter 1 (January to March) to 43 percent in Quarter 2 (April to June), with the Quarter 2 sample being skewed toward households in which people were older and more affluent (NCHS, 2021). In-person visits to households resumed in selected areas in July 2020 and in all areas of the country in September 2020. However, NHIS used a “telephone-first” approach, in which contact was first attempted by telephone, and in-person contact was attempted only to follow up with nonrespondents, to deliver recruitment materials, or to contact households when telephone numbers were unknown. The household response rate was 49 percent in Quarter 3 (July to September 2020) and approximately 54 percent in Quarter 4 (October to December). NHIS expects to continue this telephone-first approach for the foreseeable future (NCHS, 2021).
From August through the end of December 2020, the NHIS also included a longitudinal component with a subsample of about 20,000 adult respondents who completed the 2019 NHIS; this subsample had known representativeness and nearly complete telephone contact information. The longitudinal component was intended to provide data from the same individuals on health, health care, and well-being before and during the COVID-19 pandemic. The completion rate for this longitudinal follow-up survey was between 50 and 60 percent.
Thus, the 2020 NHIS included four separate designs: (1) in-person data collection in Quarter 1 (following the same procedures as in prior years), (2) telephone-only data collection in Quarter 2, (3) telephone-first data collection in Quarters 3 and 4, and (4) longitudinal follow-up in August to December. The samples for Quarters 3 and 4 also were reduced by approximately half to make resources available for the longitudinal follow-up. As for the 2020 NSDUH, the task at hand for the NHIS is determining how to use weighting and estimation techniques to produce official 2020 estimates from the disparate pieces, each of which has its own coverage and nonresponse issues (NCHS, 2021).
Before the COVID-19 pandemic, the NHIS estimate of current cigarette use has tended to be lower than the NSDUH estimate. In 2017, for example, NSDUH and NHIS estimates of current cigarette use among adults were 19.4 and 14.0 percent, respectively (CBHSQ, 2018a; NCHS, 2018b). Similar to BRFSS, adults in the NHIS are defined as current cigarette users if they smoked at least 100 cigarettes in their lifetime and reported that they currently smoke. Therefore, lower estimates of current cigarette use in the NHIS than in NSDUH could partly be explained by the different NHIS definition.
However, the NHIS definition of binge alcohol use for adults is not comparable with the NSDUH definition. In the NHIS, consumption of five or more drinks on at least 1 day is measured for the past year. For NSDUH, the reference period is the past 30 days.
Another methodological difference that can also affect comparability between NSDUH and NHIS estimates is the mode of question administration. As noted previously, sensitive questions in NSDUH are self-administered using ACASI, whereas NHIS questions are interviewer administered using CAPI or through telephone interviews beginning in March 2020. The principal focus of NSDUH on substance use and mental health issues versus the presentation of substance use questions in the NHIS in the context of a broader array of health issues also could contribute to differences in estimates.
In a separate study of 140 cognitive interview respondents in eight cities, Miller (2019) found that participants made errors in responding to NHIS questions on opioid use or NSDUH questions on prescription pain reliever use. An example of a “false positive” error was considering a nonopioid medication to be an opioid. Examples of “false negative” errors included not realizing that a medication respondents took was an opioid or not knowing the name of specific opioids they took. However, the error patterns differed across the NHIS and NSDUH questions due to differences in question wordings, how the questions were presented, and the specific terms used.
For further details, see the NHIS website at https://www.cdc.gov/nchs/nhis/index.htm.
5.1.8 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC)
The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) was preceded by the National Longitudinal Alcohol Epidemiologic Survey (NLAES). The NLAES was conducted in 1991 and 1992 by the U.S. Census Bureau for the National Institute on Alcohol Abuse and Alcoholism (NIAAA), which is part of NIH. Despite the survey name, the NLAES design was cross-sectional. NESARC’s first wave was conducted in 2001 and 2002, also by the U.S. Census Bureau for NIAAA. NESARC’s second wave was conducted in 2004 and 2005, involving reinterviews of respondents from the first wave (Grant & Dawson, 2006; NIAAA, 2010).
NESARC-III, conducted from April 2012 through June 2013, was the most recent cross-sectional survey based on a nationally representative sample of the civilian, noninstitutionalized population of the United States aged 18 years or older, including adults in all 50 states and the District of Columbia and adults living in noninstitutional group quarters. Black, Hispanic, and Asian adults were oversampled to allow reliable estimates to be made for these groups. The survey was conducted by Westat for NIAAA using CAPI. The final sample size of adults was 36,309, including adults living in households and in selected noninstitutional group quarters (Grant et al., 2015).
NESARC contained assessments of alcohol, tobacco, and other drug use, as well as dependence and abuse and certain mental disorders. NESARC-III used the Alcohol Use Disorder and Associated Disabilities Interview Schedule-5 (AUDADIS-5) to assess SUD based on criteria from the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (APA, 2013; Hasin et al., 2015). Mood disorders assessed in NESARC included major depressive disorder (MDD), dysthymia, bipolar I disorder, and bipolar II disorder. Anxiety disorders that were assessed included panic disorder (with or without agoraphobia), social phobia, specific phobia, and generalized anxiety disorder (Grant et al., 2004). An additional component of NESARC-III was collection of saliva samples from consenting respondents to obtain DNA.
A number of methodological factors could contribute to differences in estimates between NSDUH and NESARC. Questions about sensitive topics in NSDUH are self-administered (in person or via the web in 2020), and similar questions were interviewer administered in NESARC. In addition, differences in instrumentation for measurement of substance use, SUDs, and mental disorders, including differences in item sequencing and the context of questions, could affect the comparability of prevalence estimates between the two surveys (e.g., AUDADIS-5 for NESARC-III and depression questions adapted from the NCS-R for NSDUH).
For further details, see the NESARC-III website at https://www.niaaa.nih.gov/research/nesarc-iii. Also, see the 2019 NSDUH methodological summary and definitions report (CBHSQ, 2020c) for additional details.
5.1.9 National Longitudinal Study of Adolescent Health (Add Health)
The National Longitudinal Study of Adolescent Health (Add Health) was conducted to measure the effects of family, peer group, school, neighborhood, religious institution, and community influences on health risks, such as tobacco, drug, and alcohol use. Add Health was initiated in 1994 and supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from 23 other federal agencies and foundations.
The study began in 1994-1995 (Wave I) with an in-school questionnaire administered to a nationally representative sample of 90,000 students in grades 7 to 12 in 144 schools and followed up with an in-home interview. In Wave I, the students were administered brief, machine-readable questionnaires during a regular class period. Interviews also were conducted with about 20,000 students and their parents in the students’ homes using a combined CAPI and ACASI design. In Wave II, conducted in 1996, about 15,000 students in grades 8 to 12 were interviewed a second time in their homes. In Wave III in 2001-2002, about 15,000 of the original Add Health respondents, then aged 18 to 26, were reinterviewed to investigate how adolescent experiences and behaviors are related to outcomes during the transition to adulthood. Wave IV was conducted in 2007-2008 when the approximately 15,000 respondents were aged 24 to 32. Add Health reinterviewed cohort members in a Wave V follow-up from 2016 to 2018 using a mixed-mode survey design using web-based or paper-and-pencil questionnaires (Harris et al., 2019). Wave V collected social, environmental, behavioral, and biological data with which to track the emergence of chronic disease as the cohort moves through their fourth decade of life. Wave V analyses also aim to establish causes of death among study participants who have died since the start of the study. A restricted-use data file has been released containing data from 3,872 of the Wave V sample 1 respondents; a public use file of the Wave V sample 1 respondent data will not be released.
The study provides information on the use of alcohol, illicit drugs, and tobacco and has measured SUDs in some waves of the study. The longitudinal design of Add Health, in which the same sample of respondents is followed over time (and is subject to attrition in later waves of the survey), limits the kinds of comparisons that can be made with cross-sectional NSDUH data, in which estimates are based on independent samples. However, Add Health’s longitudinal design can allow data users to understand temporal relationships in ways that NSDUH’s cross-sectional design cannot (e.g., whether substance use in an earlier wave predicts another outcome in a later wave).
Another factor that affects comparability of Add Health and NSDUH data is differences in measures. For example, binge alcohol use for Add Health has been defined as having five or more drinks in one setting more than once a month in the past year (Humensky, 2010). Since 2015, NSDUH has defined binge alcohol use in terms of consumption of four or more drinks for females or five or more drinks for males on 1 or more days in the past month, regardless of the frequency of this behavior in the past year. Also, estimates of alcohol dependence or abuse have been reported for the lifetime period for Add Health (Haberstick et al., 2014). In NSDUH, the estimates are measured for the past year.
For further details, see the Add Health website at https://www.cpc.unc.edu/projects/addhealth  .
.
5.1.10 National Survey of Children’s Health (NSCH)
Since 2001, the Health Resources and Services Administration’s (HRSA’s) Maternal and Child Health Bureau (MCHB) has sponsored the National Survey of Children’s Health (NSCH) and its companion survey, the National Survey of Children with Special Health Care Needs (NS-CSHCN). These studies were designed to provide national- and state-level prevalence estimates for a variety of physical health, substance use, and mental health indicators among children aged 0 to 17 in the United States. The NSCH was previously conducted in 2003, 2007, and 2011-2012; the NS-CSHCN was conducted three times between 2001 and 2010 (2001, 2005-2006, and 2009-2010). The surveys were conducted as modules of the State and Local Area Integrated Telephone Survey (SLAITS) system by the NCHS. SLAITS used random-digit-dialing sampling of landline telephone numbers, with cellular telephone supplementation in the last year of administration for both surveys. In 2015, the MCHB redesigned the NSCH and NS-CSHCN into a single combined survey, incorporating questions from both surveys and retaining the NSCH name. The most recent available data since the redesign took place are from 2019, with data collection conducted by the U.S. Census Bureau on behalf of the NCHS.
The redesigned survey uses an address-based sampling frame in which addresses are randomly sampled within states. Administrative records are then used to identify households likely to have children. Households are sampled according to their likelihood of containing children. Data are collected on one child per household, with children with special health care needs and children aged 5 or younger having a higher probability of selection. A parent is asked to provide data on the sampled child.
The principal household screening and survey mode is via web-based instruments, but data can be collected using alternate modes (i.e., over the telephone or using mailed paper-and-pencil instruments) if parents prefer not to complete the survey online. Screening and survey instruments are available in English and Spanish.127 The NSCH results are weighted to represent the population of noninstitutionalized children aged 0 to 17 years nationally and in each state (U.S. Census Bureau, 2020).
The parent completing the survey was asked whether a doctor or other health professional ever told the parent that the child had specific mental health conditions, including depression. If the parent reported being told that the child ever had depression, the parent was asked whether the child currently has depression and, if so, whether the adult would describe the child’s depression as mild, moderate, or severe. Estimates for depression from the combined 2018 and 2019 NSCH were presented for children aged 3 to 17. Based on combined 2018 and 2019 NSCH data, the estimated prevalence of current depression nationally among adolescents aged 12 to 17 was 7.2 percent, and 3.8 percent of adolescents were described as currently having moderate or severe depression.128 The 2019 NSDUH estimate of MDE in the past year among adolescents aged 12 to 17 was 15.7 percent, and 11.1 percent had MDE with severe impairment (CBHSQ, 2020a).
Methodological differences between NSDUH and the NSCH for 2019 that could affect the estimates of depression among adolescents include the following: (1) the modes of administration (ACASI for NSDUH129 vs. multimode administration [web, telephone, or paper-and-pencil] for the NSCH); (2) the source of information about an adolescent’s health (direct self-reports from adolescent respondents in NSDUH vs. parental reports in the NSCH); (3) differences in measures for estimating the prevalence and severity of depression;130 and (4) differences in the reference period for recent depression (past 12 months in NSDUH vs. “currently” in the NSCH). The weighted response rate for adults in the 2019 NSDUH was 64.2 percent (CBHSQ, 2020c).131 The overall weighted response rate for the 2019 NSCH was 42.4 percent (U.S. Census Bureau, 2020). Thus, differential nonresponse bias patterns also could contribute to differences in estimates between these two surveys.
For further details, see the NSCH website at https://www.childhealthdata.org/learn-about-the-nsch/NSCH  .
.
5.1.11 National Youth Tobacco Survey (NYTS)
The National Youth Tobacco Survey (NYTS) is a cross-sectional, voluntary, school-based, self-administered electronic survey of middle and high school students in the United States. The NYTS is designed to provide national data on long-term, intermediate, and short-term indicators key to the design, implementation, and evaluation of comprehensive tobacco prevention and control programs. The survey uses a stratified three-stage cluster sampling procedure to generate a nationally representative sample of students attending public and private schools in grades 6 through 12. Participants complete the survey in classrooms using a tablet computer. In 2020, data collection occurred from January 16 through March 16, 2020, but was cut short because of widespread school closures during the COVID-19 pandemic (instead of data collection occurring through May 2020). In total for 2020, 14,531 students participated from 180 schools, for a student participation rate of 87.4 percent, a school participation rate of 49.9 percent, and an overall response rate of 43.6 percent (i.e., the product of the school and student participation rates) (Gentzke et al., 2020).
In general, school-based surveys such as the NYTS are likely to provide higher estimates of tobacco use for youths compared with estimates in NSDUH. For example, the NYTS estimated that in the past 30 days, 16.2 percent of middle and high school students in 2019 used any tobacco product (defined as the use of e-cigarettes, cigarettes, cigars, smokeless tobacco, hookahs, pipe tobacco, bidis [small brown cigarettes wrapped in a leaf], or heated tobacco products), 13.1 percent used e-cigarettes, and 3.3 percent used cigarettes (Gentzke et al., 2020). Estimates of use in the past 30 days from the 2020 NSDUH for youths aged 12 to 17 were 6.5 percent for tobacco products or nicotine vaping, 5.1 percent for nicotine vaping, and 1.4 percent for cigarettes (CBHSQ, 2021h). Although measures were not exactly comparable between the two surveys (e.g., respondents in the 2020 NSDUH were not asked about the use of hookahs or bidis), lower prevalence estimates in household surveys such as NSDUH than in school-based surveys may be due to more underreporting in household settings and some overreporting in school settings (Fowler & Stringfellow, 2001). Consistent with 2020 NYTS estimates, however, nicotine vaping was the most common form of nicotine product use among youths in the 2020 NSDUH.
Similar to other school-based surveys, the population of inference for the NYTS is adolescents who are in middle school or high school, specifically those in the 6th through 12th grades. Consequently, the NYTS does not include data from adolescents who have dropped out of school. For these reasons, NYTS data cannot be used for making inferences about the adolescent population of the United States as a whole.
For further details, see https://www.fda.gov/tobacco-products/.
5.1.12 Youth Risk Behavior Survey (YRBS)
Since 1991, the national Youth Risk Behavior Survey (YRBS) has been a component of the CDC’s Youth Risk Behavior Surveillance System (YRBSS), which measures the prevalence of six priority health risk behavior categories: (1) behaviors that contribute to unintentional injuries and violence; (2) tobacco use; (3) alcohol and other drug use; (4) sexual behaviors related to unintended pregnancy and sexually transmitted diseases, including human immunodeficiency virus infection; (5) unhealthy dietary behaviors; and (6) physical inactivity. The YRBSS includes state, territorial, tribal, and local school-based surveys of high school students conducted every 2 years. The national school-based survey uses a three-stage cluster sample design to produce a nationally representative sample of students in grades 9 through 12 who attend public and private schools. The national YRBS is conducted biennially during the spring, with students completing a self-administered, machine-readable questionnaire during a regular class period. For the 2019 national YRBS, 13,872 questionnaires were completed in 136 schools (Underwood et al., 2020).
In general, the YRBS school-based survey has found higher rates of substance use for youths than those found in NSDUH (Tables 5.1 and 5.3). The lower prevalence rates in NSDUH are likely due to the differences in study design (Fowler & Stringfellow, 2001). As in the case of comparisons with estimates from MTF and other school-based surveys, the lower estimates in NSDUH may be due to more underreporting in the household setting, as compared with the YRBS school setting, and to some overreporting in the school settings (CBHSQ, 2012b). As for MTF, most substance use questions in the YRBS ask respondents to report the number of times they have used a substance; this frequency-of-use question format could increase the reports of substance use (including potential overreporting) compared with the “yes/no” question format in NSDUH (other than prescription drugs) that allows respondents to skip remaining questions about that substance if they do not report lifetime use. The principal focus of NSDUH on substance use and mental health issues versus the presentation of substance use questions in the YRBS in the context of a broader array of health issues also could contribute to differences in estimates.
Similar to other school-based surveys, the population of inference for the YRBS is the population of adolescents who are in school, specifically those in the 9th through 12th grades. Consequently, the YRBS does not include data from dropouts. The YRBS makes follow-up attempts to obtain data from youths who were absent on the day of survey administration but nevertheless does not obtain complete coverage of these youths. For these reasons, YRBS data are not intended to be used for making inferences about the adolescent population of the United States as a whole.
For further details, see the YRBS website at https://www.cdc.gov/healthyyouth/data/yrbs/index.htm.
5.2 Substance Use Treatment Data Sources
SAMHSA’s Behavioral Health Services Information System (BHSIS) includes three components that provide national- and state-level information on the numbers and characteristics of individuals admitted to substance use treatment programs and that describe the facilities that deliver care to those individuals. The core of BHSIS is the Inventory of Behavioral Health Services (I-BHS), a comprehensive listing of all known substance use treatment and mental health care facilities. The focus of I-BHS is to update information continually; therefore, summary statistics about I-BHS are not included in this section. The two other components of BHSIS are described in this section: the National Survey of Substance Abuse Treatment Services (N-SSATS) and the Treatment Episode Data Set (TEDS).
5.2.1 National Survey of Substance Abuse Treatment Services (N-SSATS)
The National Survey of Substance Abuse Treatment Services (N-SSATS) started in 2000 and is an annual census of all known drug and alcohol abuse treatment facilities in the United States and U.S. jurisdictions. The N-SSATS currently employs three sequential data collection modes: a secure web-based questionnaire, a paper questionnaire sent by mail upon request to facilities that had not responded to the web-based questionnaire, and a telephone interview for facilities that had not responded to the web or paper questionnaire. Most facilities complete the web-based questionnaire. For the 2019 N-SSATS, the response rate among eligible facilities was 91.4 percent. Among the responding facilities, 89.8 percent completed the survey via the web (CBHSQ, 2020f).
In N-SSATS, facilities provide information on the characteristics of the treatment facility, including (but not limited to) client payment sources, services provided, and hospital and residential capacity. In addition, N-SSATS collects data from facilities on the number of clients in treatment on the survey reference date (i.e., the last working day of March in the survey year, such as March 31, 2019) and the percentages of clients in treatment on the reference date for abuse of alcohol and other drugs, alcohol abuse only, other drug abuse only, and co-occurring SUDs and mental disorders.
In an analysis comparing NSDUH and N-SSATS data, average counts of the number of people in treatment for alcohol or illicit drug abuse on a single day were about 1.2 million based on N-SSATS data from 2007 to 2009. Corresponding average single-day counts from NSDUH were about 1.4 million based on the questionnaire item asking about treatment on October 1 and 1.2 million based on the item about currently being in treatment at the time of the interview.132 Compared with facilities responding to N-SSATS, NSDUH respondents were more likely to report treatment only for alcohol and were less likely to report treatment only for illicit drugs (Batts et al., 2014).
As noted previously, N-SSATS collects data on substance use treatment utilization from facilities. In contrast, NSDUH estimates of treatment utilization are based on self-reports of treatment from respondents in the general population. The validity of N-SSATS data on treatment utilization depends on the accuracy of the reports provided by the individual(s) responding on behalf of the facility just as the validity of NSDUH estimates on the receipt of substance use treatment depends on accurate respondent self-reports. Also, N-SSATS counts of clients who received treatment cover clients who may be outside of the NSDUH target population (e.g., homeless people not living in shelters, active-duty military personnel). In addition, N-SSATS percentages of clients receiving treatment both for alcohol and other drugs, only alcohol, and only other drugs are based on responses to a single question that asks a facility staff member to assign these percentages to each category. In contrast, NSDUH respondents who reported receiving treatment at a specialty facility are asked about the substances for which they received treatment.
For further details, see the SAMHSA website at https://www.samhsa.gov/data/.
5.2.2 Treatment Episode Data Set (TEDS)
The Treatment Episode Data Set (TEDS) is a compilation of data on the demographic characteristics and substance abuse problems of those aged 12 or older who are admitted for substance use treatment, based on administrative data routinely collected by state substance abuse agencies (SSAs) for substance abuse services. SSAs report data to TEDS for approximately 2 million annual admissions to treatment in the United States and Puerto Rico primarily from facilities receiving some public funding. The TEDS system consists of two major components—the Admissions Data Set and the Discharge Data Set. The TEDS Admissions Data Set includes annual client-level data on substance use treatment admissions since 1992. The TEDS Discharge Data Set can be linked at the record level to admissions and includes information from clients discharged in 2000 and later. The most current TEDS data at the time this report was written were the 2018 admissions data and discharge data from publicly funded treatment (CBHSQ, 2020g).
The TEDS Admissions Data Set consists of a Minimum Data Set collected by all states and a Supplemental Data Set collected by some states. The Minimum Data Set for 2018 consisted of 19 items that include demographic information; primary, secondary, and tertiary substance problems at admission; source of referral; number of prior treatment episodes; and service type at admission, including planned use of medication-assisted opioid therapy. Supplemental Data Set items for 2018 consisted of 15 items that include psychiatric, social, and economic measures. The TEDS Discharge Data Set consists of items on service type at discharge, reason for discharge (e.g., completed treatment, transferred to another program or facility, dropped out), and length of stay (LOS). LOS is calculated by subtracting the admission date from the discharge date (or date of last contact).
In an analysis comparing NSDUH and TEDS data that included linked admissions and discharge data from TEDS, the average number of individuals who received treatment in the past year based on TEDS data from 2007 to 2009 was about 22 percent lower than the average from 2005 to 2010 in NSDUH for treatment in a specialty facility (1.9 million vs. 2.4 million). TEDS also yielded a lower number of individuals in treatment on a single day (0.5 million in 2007 to 2009) compared with single-day counts for N-SSATS (1.2 million) and NSDUH (1.2 million to 1.4 million, depending on the questions that were used; see the N-SSATS description in this section) (Batts et al., 2014).133
An important issue for users of NSDUH and TEDS data to consider is that the unit of analysis for TEDS is admissions to substance use treatment, whereas NSDUH estimates are for individuals who received substance use treatment. Consequently, individuals who were admitted to substance use treatment multiple times in the reporting period would be counted more than once in the TEDS admissions data. In addition, TEDS includes data for a sizable proportion of admissions to substance use treatment, but it does not include all admissions. Because TEDS is a compilation of data from state administrative systems, the scope of facilities included in TEDS is affected by differences in state reporting requirements, licensure, certification, and accreditation practices, as well as disbursement of public funds. Many SSAs require facilities that receive public funding (including federal block grant funds) for substance use treatment services to report data to the SSA, whereas others require all facilities that are licensed or certified by the state to report TEDS data. States also vary in terms of the specific admissions that are reported to TEDS (e.g., all admissions to eligible facilities that report to TEDS vs. admissions financed by public funds).
For further details, see the SAMHSA website at https://www.samhsa.gov/data/.
5.3 Surveys of Populations Not Covered by NSDUH
Although the civilian, noninstitutionalized population covers the large majority of people in the United States (Lofquist et al., 2012), it excludes some subpopulations that may have very different estimates of mental disorders and substance use and therefore may have specific issues or needs. Specifically, the civilian, noninstitutionalized population does not include active-duty military personnel or people living in institutional group quarters, such as prisons, residential substance use treatment or mental health facilities, nursing homes, and long-term hospitals.
This section includes sources of national-level data for members of these subpopulations. As noted in this chapter’s introduction, demographic differences between the civilian, noninstitutionalized population and these other populations can contribute to differences in substance use and mental health outcomes. Therefore, this section does not compare estimates between populations unless analyses have adjusted for demographic differences between the populations.
5.3.1 Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS)
The Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS) is a multicomponent epidemiologic and neurobiological study to inform health promotion, risk reduction, and suicide prevention efforts in the U.S. Army. A primary aim of the study is to increase knowledge about determinants of suicidal thoughts and behaviors among soldiers. Army STARRS is supported through the Henry M. Jackson Foundation for the Advancement of Military Medicine under a cooperative agreement between NIMH and a consortium of scientific collaborators at the Uniformed Services University of the Health Sciences, the University of California San Diego, Harvard Medical School, and the University of Michigan, with additional collaborating scientists and consultants from NIMH and the Army. Data collection was completed in 2015.
Army STARRS had eight component studies that included the use of administrative data and self-reports from surveys: (1) the Historical Administrative Data Study (HADS), an integrated analysis of Army and Department of Defense (DoD) administrative data systems to provide data on predictors of suicides among soldiers who were on active duty during 2004 through 2009; (2) the New Soldier Study (NSS), a cross-sectional survey in 2011 and 2012 of new soldiers in the 2 days after their arrival for Basic Combat Training (BCT); (3) the All-Army Study (AAS), a cross-sectional survey in 2011 through 2013 of active-duty personnel other than those in BCT; (4) the Soldier Health Outcomes Study A, a retrospective case-control study in 2011 through 2013 of soldiers who made nonfatal suicide attempts; (5) the Soldier Health Outcomes Study B, a case-control study in 2012 through 2014 focusing on soldiers whose suicide attempts were fatal; (6) the Pre-Post Deployment Survey, in which NSS and AAS respondents were tracked longitudinally from 2012 to 2014 through their administrative records to obtain information on outcomes related to treatment for mental illness, nonfatal suicide attempts, and suicide fatalities; (7) the Criminal Investigation Division (CID) Study, which involved a review of Army CID file reports for deaths of Army service members in 2005 to 2009 due to suicide, accident, traffic fatality, justifiable homicide, or undetermined cause; and (8) the Clinical Reappraisal Study, which was designed to validate and calibrate the mental disorder screening tools in the Army STARRS questionnaire. More information about these component studies can be found in Kessler et al. (2013) and on the website listed at the end of this section.
The questionnaires for both the NSS and AAS were self-administered in group sessions and collected information on physical health (including periods of insomnia and chronic pain); internalizing mental disorders (e.g., MDD, bipolar disorder, panic disorder, generalized anxiety disorder [GAD], posttraumatic stress disorder [PTSD], specific phobia, social phobia, obsessive-compulsive disorder); externalizing mental disorders (e.g., attention-deficit/hyperactivity disorder [ADHD], conduct disorder, intermittent explosive disorder [IED], oppositional defiant disorder [ODD], SUD) (Nock et al., 2014; Rosellini et al., 2015); receipt of mental health services; substance use; and suicidal thoughts and behaviors (Nock et al., 2014; Ursano et al., 2015). Assessment of mental disorders or SUDs was based on DSM-IV criteria for the lifetime, past 12-month, and past 30-day periods, except that disorders were assessed without regard to diagnostic hierarchy or organic exclusion rules (Kessler et al., 2014). The NSS questionnaire used computer-assisted self-interviewing (CASI) and was administered on laptop computers. The AAS questionnaire was shorter than the NSS questionnaire (i.e., designed for a single 90-minute group administration instead of two 90-minute administrations for the NSS), and it was designed for CASI administration or as a paper-and-pencil questionnaire. In addition, the NSS included neurocognitive tests and blood samples for genetic testing that were obtained from consenting participants as part of the physical examination process prior to the beginning of BCT. The AAS did not collect neurocognitive data or physical specimens for genetic testing. Both NSS and AAS respondents were asked for additional consent to link their Army or DoD administrative records to their questionnaire responses and to participate in to-be-determined future longitudinal data collections (Kessler et al., 2013).
Component studies from Army STARRS have documented the prevalence of mental disorders among military personnel, including conditions with an onset before personnel enlisted. For example, AAS data from 5,428 soldiers indicated that 25.1 percent of respondents met criteria for any mental disorder or SUD in the past 30 days, including 15.0 percent for any internalizing disorder (bipolar disorder, GAD, MDD, panic disorder, or PTSD), 18.4 percent for any externalizing disorder (ADHD, conduct disorder, IED, ODD, or SUD), and 11.1 percent for multiple disorders (internalizing or externalizing). About three fourths of respondents with any disorder in the past 30 days (76.6 percent) reported an age at onset prior to enlistment (Kessler et al., 2014). Lifetime estimates for suicidal thoughts and behaviors were 13.9 percent for having suicidal thoughts, 5.3 percent for making a suicide plan, and 2.4 percent for making a (nonfatal) suicide attempt (Nock et al., 2014). Among soldiers with a mental disorder in the past 30 days who did not seek treatment, 69.8 percent did not perceive a need for treatment. Among soldiers with a mental disorder who perceived a need for treatment, attitudinal reasons (e.g., wanting to handle the problem on their own) were cited more commonly than structural reasons (e.g., inconvenience) for not seeking or for discontinuing treatment (Naifeh et al., 2016).
NSS data from 38,507 new soldiers indicated that 38.7 percent of new soldiers had 1 or more of the 10 assessed DSM-IV disorders in their lifetime, including 19.8 percent who had an internalizing disorder (bipolar disorder, GAD, MDD, panic disorder, or PTSD) and 31.8 percent who had an externalizing disorder (ADHD, conduct disorder, IED, ODD, or SUD). Comparison of NSS estimates with NCS-R estimates that controlled for demographic differences between the NSS and civilian populations134 indicated similar overall estimates of any lifetime disorder in the two populations. However, new soldiers were more likely than adults in the general civilian population to have GAD, PTSD, conduct disorder, or multiple (i.e., three or more) disorders in their lifetime (Rosellini et al., 2015). NSS also yielded lifetime pre-enlistment estimates of 14.1 percent for suicidal thoughts, 2.3 percent for suicide plans, and 1.9 percent for suicide attempts (Ursano et al., 2015).
Administrative data from the HADS component for more than 743,000 reserve component (RC) personnel who had been activated from January 1, 2006, through December 31, 2009, identified 1,103 soldiers with a documented suicide attempt. Activated RC soldiers who were enlisted accounted for 95.7 percent of the activated RC soldiers with a suicide attempt. Officers accounted for the remaining 4.3 percent of the activated RC soldiers who attempted suicide. Among enlisted RC personnel who had been activated, predictors of suicide attempts included the following: female gender, current age younger than 30, white, non-Hispanic race/ethnicity, less than a high school education, currently being married, time in service of 1 to 2 years, previous deployment, and a history of a mental health diagnosis. Predictors of suicide attempts among corresponding RC officers included being female and having a mental health diagnosis in the previous month (Naifeh et al., 2019).
Additionally, a new data collection, the STARRS Longitudinal Study (STARRS-LS), is under way. The STARRS-LS began in 2015 and has been approved to continue through 2024. The STARRS-LS is gathering longitudinal follow-up information on soldiers who participated in Army STARRS earlier in their Army careers and as they transition back into civilian life.
For further details, see the Army STARRS-LS website at https://starrs-ls.org/#/  .
.
5.3.2 Minimum Data Set (MDS)
The Minimum Data Set (MDS), sponsored by the Centers for Medicare & Medicaid Services (CMS), is part of the federally mandated process for clinical assessment of all residents in Medicare- or Medicaid-certified nursing homes. This process provides a comprehensive assessment of each resident’s functional capabilities and helps nursing home staff identify health problems. MDS assessments are completed on admission, periodically, and at discharge for all residents in certified nursing homes, regardless of source of payment for the individual resident, and within specific guidelines and time frames. MDS assessments are completed every 3 months (or more often, depending on circumstances) on nearly all residents of nursing homes in the United States. In most cases, participants in the assessment process are licensed health care professionals employed by the nursing home. MDS information is transmitted electronically by nursing homes to the national MDS database at CMS. Thus, unlike many of the sources of data described in this section of the report, MDS data are not based on survey results.
Selected psychiatric diagnoses for active residents are summarized quarterly in the MDS 3.0 Frequency Report; no substance use information is available, and data are not summarized annually. The unit of reporting is an active resident135 or a resident with an active episode. The MDS items are taken from all types of MDS records, with the most recent value in the episode being taken for each item. Only values from the past 440 days are used for all items, except for items from the initial admission record. Thus, different items may come from different assessments or from different stays within an episode of care. The intention is to create a profile with the most recent standard information for an active resident, regardless of the source of information. Percentages of active residents are based on data from nearly 1.4 million active residents nationally; records with missing data for a given measure were excluded.
Substantial percentages of active residents had psychiatric diagnoses in the past 7 days. In each quarter of 2019, for example, nearly half of active residents were diagnosed as having depression other than bipolar disorder. Nearly 1 in 3 residents had an anxiety disorder. About 1 in 10 residents had schizophrenia, and about 1 in 12 had a psychotic disorder other than schizophrenia.
For further details about the MDS, see the “Research, Statistics, Data & Systems” page on the CMS website at https://www.cms.gov/. Publicly available quarterly data from the MDS 3.0 Frequency Report can be accessed on the web.
5.3.3 National Inmate Surveys (NIS)
The National Inmate Surveys (NIS) were initiated to fulfill the requirements of the Prison Rape Elimination Act of 2003 (PREA, 2003) for the Bureau of Justice Statistics (BJS) to provide a list of prisons and jails according to the prevalence of sexual victimization. The BJS also added a companion survey on drug and alcohol use and treatment as part of the NIS. Inclusion of the companion survey was designed to prevent facility staff from knowing whether inmates were selected to receive the survey on sexual victimization or the companion survey and was intended to provide more recent information on substance use and related issues among correctional populations in the United States compared with the Surveys of Inmates in State and Federal Correctional Facilities. The NIS were conducted in 2007 (NIS-1), in 2008-2009 (NIS-2), and in 2011-2012 (NIS-3). Questions about mental health were included for the first time in the NIS-3.
The NIS used a two-stage probability sample design first to select state and federal correctional facilities136 and then to select inmates within sampled facilities. At least one facility in every state was selected.137 The sample design also ensured a sufficient number of women in the sample. Samples were restricted to confinement facilities (i.e., institutions in which fewer than 50 percent of the inmates were regularly permitted to leave for work, study, or treatment without being accompanied by facility staff). The NIS samples also excluded community-based facilities, such as halfway houses, group homes, and work release centers. Inmates aged 18 or older within sampled facilities were randomly selected for the interview.
The NIS-1 was conducted in 146 state and federal prisons and in 282 local jails between April and August 2007 and obtained the drug and alcohol survey from 7,754 prison or jail inmates. The NIS-2 was conducted in 167 state and federal prisons and 286 jails between October 2008 and August 2009 and obtained the drug and alcohol survey from 5,015 prison or jail inmates. The NIS-3 was conducted in 233 state and federal prisons, 358 local jails, and 15 special facilities (military, Indian country, and U.S. Immigration and Customs Enforcement) between February 2011 and May 2012. A total of 106,532 inmates participated in NIS-3 (either survey form), including 43,721 state or federal prison inmates, 61,351 jail inmates, and 1,460 inmates in special facilities (Beck et al., 2013).
The interviews used CAPI for general background information at the beginning of the interview and ACASI for the remainder. Respondents completed the ACASI portion of the interview in private, with the interviewer either leaving the room or moving away from the computer.138 Sampled inmates were randomly assigned to receive the sexual victimization survey or the companion survey on substance use and treatment. Substance use questions were based on items from past inmate surveys conducted by BJS, such as the 2004 Survey of Inmates in State Correctional Facilities, and included questions about lifetime and first use of drugs or alcohol, being under the influence of drugs or alcohol at the time of their current offense, substance use prior to being admitted to the facility, problems associated with substance use, and treatment for use of drugs or alcohol. The NIS-3 included questions on the following mental health issues: (1) serious psychological distress (SPD) in the past 30 days, based on the Kessler-6 (K6) questions (see Section 3.4.7.4 in this report for a list of the K6 questions); (2) occurrence of specific mental disorders in the lifetime and past 12-month periods; (3) whether respondents had ever been told that they had specific mental disorders; and (4) mental health service utilization. Similar to NSDUH, the NIS-3 defined inmates as having SPD if they had a K6 score of 13 or greater for the past 30 days (Beck et al., 2013; Bronson & Berzofsky, 2017).
NIS-1 and NIS-2 data from 2007 to 2009 indicated high rates of illicit drug use and SUDs. For example, nearly 40 percent of state inmates (39.3 percent) and more than half of sentenced jail inmates (54.5 percent) used illicit drugs in the month before their offense, including 27.5 percent of state prisoners and 38.7 percent of sentenced jail inmates who used marijuana in that period and 14.7 percent of state prisoners and 21.1 percent of sentenced jail inmates who used cocaine or crack. More than half of state prisoners (58.5 percent) and nearly two thirds of sentenced jail inmates (63.3 percent) met DSM-IV criteria for illicit drug use disorder (i.e., dependence or abuse), defined for the survey according to the occurrence of symptoms in the year prior to their admission to their current facility. Among inmates who met criteria for an illicit drug use disorder, 28.5 percent of those who were state prisoners and 22.2 percent of those who were sentenced jail inmates received substance use treatment or participated in a program (e.g., self-help groups) since being admitted to their current facility (Bronson et al., 2017).
Analyses of the NIS substance use data from 2007 to 2009 also included comparisons with NSDUH data for adults from these years. To account for demographic differences between the general population and inmate population that also are associated with substance use, NSDUH data for adults were standardized to the state prisoner population based on gender, race, Hispanic origin, and age. Estimates for the inmate population were greater than the standardized overall adult population estimates from NSDUH for all measures of illicit drug use in the past month (for NSDUH) or in the month before criminal justice involvement (for NIS) (Bronson et al., 2017).
Similar to the NIS-1 and NIS-2, the NIS-3 data indicated high estimates of mental health issues among the incarcerated population. An estimated 36.9 percent of prison inmates and 44.3 percent of jail inmates in the NIS-3 reported having ever been told by a mental health professional that they had a mental disorder (manic depression, bipolar disorder, other depressive disorder, schizophrenia or another psychotic disorder, PTSD, or an anxiety or personality disorder). An estimated 14.5 percent of prisoners and 26.4 percent of jail inmates had SPD in the past 30 days. In comparisons of NIS-3 data with standardized adult NSDUH estimates,139 prisoners were three times as likely and jail inmates were five times as likely as adults in the general population to have SPD. Jail inmates also were more likely to have SPD compared with adults in the general population who had been arrested in the past 12 months. However, SPD estimates were similar for prisoners and adults in the general population who had been arrested in the past year (Bronson & Berzofsky, 2017).
For further details about the NIS, see the BJS’s “All Data Collections” webpage at https://www.bjs.gov/index.cfm?ty=dca.
Figure 5.1 NSDUH and MTF Past Month Alcohol Use: Among Youths; 2002-2020
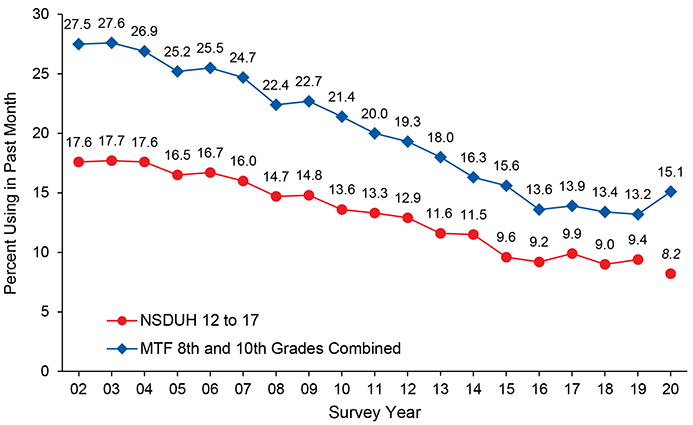 D
D
MTF = Monitoring the Future; NSDUH = National Survey on Drug Use and Health.
NOTE: The NSDUH estimate in 2020 is italicized and there is no connecting line between 2019 and 2020 to indicate caution should be used when comparing estimates between 2020 and prior years because of methodological changes for 2020. Due to these changes, significance testing between 2020 and prior years was not performed.
NOTE: MTF data collection for 2020 was halted in schools on March 15, 2020, because of the coronavirus disease 2019 (COVID-19) pandemic. The smaller 2020 sample did not differ from nationally representative results from previous years in terms of demographic characteristics and substance use prevalence estimates that had been stable in recent years (Miech et al., 2021).
Figure 5.2 NSDUH and MTF Past Month Cigarette Use: Among Youths; 2002-2020
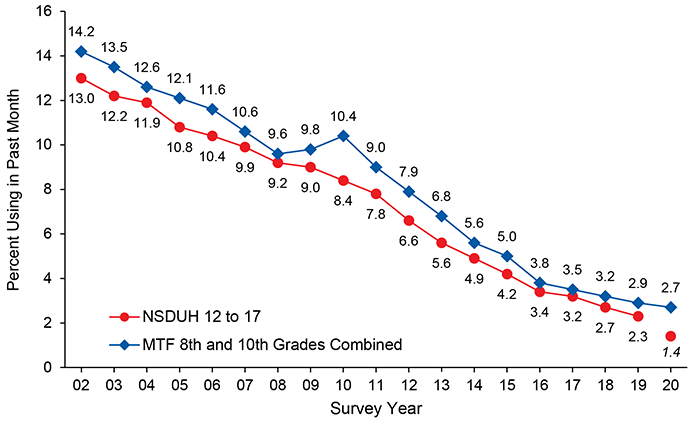 D
D
MTF = Monitoring the Future; NSDUH = National Survey on Drug Use and Health.
NOTE: The NSDUH estimate in 2020 is italicized and there is no connecting line between 2019 and 2020 to indicate caution should be used when comparing estimates between 2020 and prior years because of methodological changes for 2020. Due to these changes, significance testing between 2020 and prior years was not performed.
NOTE: MTF data collection for 2020 was halted in schools on March 15, 2020, because of the coronavirus disease 2019 (COVID-19) pandemic. The smaller 2020 sample did not differ from nationally representative results from previous years in terms of demographic characteristics and substance use prevalence estimates that had been stable in recent years (Miech et al., 2021).
Figure 5.3 NSDUH and MTF Past Month Marijuana Use: Among Youths; 2002-2020
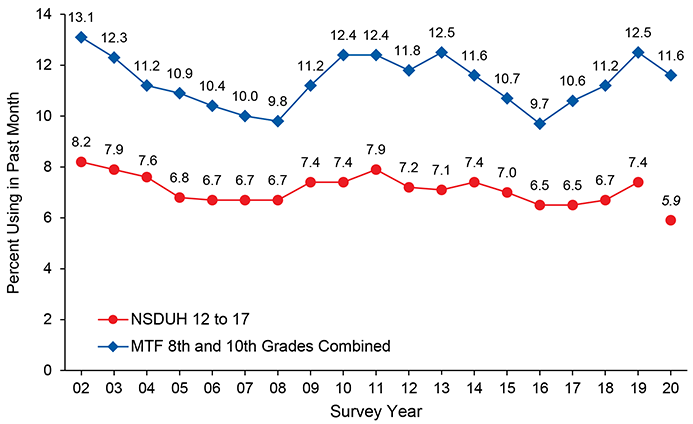 D
D
MTF = Monitoring the Future; NSDUH = National Survey on Drug Use and Health.
NOTE: The NSDUH estimate in 2020 is italicized and there is no connecting line between 2019 and 2020 to indicate caution should be used when comparing estimates between 2020 and prior years because of methodological changes for 2020. Due to these changes, significance testing between 2020 and prior years was not performed.
NOTE: MTF data collection for 2020 was halted in schools on March 15, 2020, because of the coronavirus disease 2019 (COVID-19) pandemic. The smaller 2020 sample did not differ from nationally representative results from previous years in terms of demographic characteristics and substance use prevalence estimates that had been stable in recent years (Miech et al., 2021).
Figure 5.4 NSDUH, MTF, and YRBS Past Month Marijuana Use: Among Youths; 1971 2020
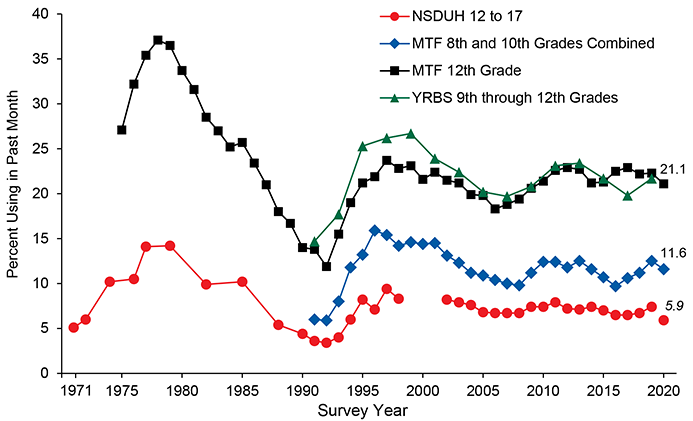 D
D
MTF = Monitoring the Future; NSDUH = National Survey on Drug Use and Health; YRBS = Youth Risk Behavior Survey.
NOTE: NSDUH data for youths aged 12 to 17 are not presented for 1999 to 2001 because of design changes in the survey. These design changes preclude direct comparisons of estimates from these years with estimates in other years. The NSDUH estimate in 2020 is italicized and there is no connecting line between 2019 and 2020 to indicate caution should be used when comparing estimates between 2020 and prior years because of methodological changes for 2020. Due to these changes, significance testing between 2020 and prior years was not performed.
NOTE: MTF data collection for 2020 was halted in schools on March 15, 2020, because of the coronavirus disease 2019 (COVID-19) pandemic. The smaller 2020 sample did not differ from nationally representative results from previous years in terms of demographic characteristics and substance use prevalence estimates that had been stable in recent years (Miech et al., 2021).
Table 5.1 – NSDUH, MTF, and YRBS Lifetime Prevalence Estimates: Among Youths; Percentages, 2002-2020
Substance/
Survey |
2002 |
2003 |
2004 |
2005 |
2006 |
2007 |
2008 |
2009 |
2010 |
2011 |
2012 |
2013 |
2014 |
2015 |
2016 |
2017 |
2018 |
2019 |
2020 |
| Marijuana |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| NSDUH |
20.6 |
19.6 |
19.0 |
17.4 |
17.3 |
16.2 |
16.6 |
17.1 |
17.1 |
17.5 |
17.0 |
16.4 |
16.4 |
15.7 |
14.8 |
15.3 |
15.4 |
15.8 |
12.4 |
| MTF |
29.0 |
27.0 |
25.7 |
25.3 |
23.8 |
22.6 |
22.3 |
24.0 |
25.4 |
25.5 |
24.5 |
26.2 |
24.7 |
23.3 |
21.3 |
22.1 |
23.3 |
24.6 |
24.1 |
| YRBS |
-- |
40.2 |
-- |
38.4 |
-- |
38.1 |
-- |
36.8 |
-- |
39.9 |
-- |
40.7 |
-- |
38.6 |
-- |
35.6 |
-- |
36.8 |
-- |
| Cocaine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| NSDUH |
2.7 |
2.6 |
2.4 |
2.3 |
2.2 |
2.2 |
1.9 |
1.6 |
1.5 |
1.3 |
1.1 |
0.9 |
0.9 |
0.8 |
0.9 |
0.7 |
0.7 |
0.6 |
0.4 |
| MTF |
4.9 |
4.4 |
4.4 |
4.5 |
4.1 |
4.2 |
3.8 |
3.6 |
3.2 |
2.8 |
2.6 |
2.5 |
2.2 |
2.2 |
1.8 |
1.7 |
2.0 |
1.9 |
1.6 |
| YRBS |
-- |
8.7 |
-- |
7.6 |
-- |
7.2 |
-- |
6.4 |
-- |
6.8 |
-- |
5.5 |
-- |
5.2 |
-- |
4.8 |
-- |
3.9 |
-- |
| Heroin |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| NSDUH |
0.4 |
0.3 |
0.3 |
0.2 |
0.2 |
0.2 |
0.3 |
0.2 |
0.2 |
0.3 |
0.2 |
0.2 |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
* |
| MTF |
1.7 |
1.6 |
1.6 |
1.5 |
1.4 |
1.4 |
1.3 |
1.4 |
1.3 |
1.2 |
1.0 |
1.0 |
0.9 |
0.6 |
0.6 |
0.6 |
0.5 |
0.6 |
0.4 |
| YRBS |
-- |
3.3 |
-- |
2.4 |
-- |
2.3 |
-- |
2.5 |
-- |
2.9 |
-- |
2.2 |
-- |
2.1 |
-- |
1.7 |
-- |
1.8 |
-- |
| LSD |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| NSDUH |
2.7 |
1.6 |
1.2 |
1.1 |
0.9 |
0.8 |
1.1 |
1.0 |
0.9 |
0.9 |
1.0 |
0.9 |
1.2 |
1.3 |
1.2 |
1.5 |
1.3 |
1.6 |
1.3 |
| MTF |
3.8 |
2.8 |
2.3 |
2.2 |
2.2 |
2.3 |
2.3 |
2.4 |
2.4 |
2.3 |
2.0 |
2.1 |
1.9 |
2.2 |
2.2 |
2.2 |
2.1 |
2.6 |
3.0 |
| YRBS |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
| Alcohol |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| NSDUH |
43.4 |
42.9 |
42.0 |
40.6 |
40.4 |
39.5 |
38.6 |
38.4 |
35.4 |
34.5 |
32.4 |
30.8 |
29.6 |
28.4 |
27.0 |
27.1 |
26.3 |
26.7 |
22.8 |
| MTF |
57.0 |
55.8 |
54.1 |
52.1 |
51.0 |
50.3 |
48.6 |
47.9 |
47.0 |
44.6 |
41.8 |
40.0 |
38.1 |
36.6 |
33.1 |
32.7 |
33.3 |
33.8 |
36.0 |
| YRBS |
-- |
74.9 |
-- |
74.3 |
-- |
75.0 |
-- |
72.5 |
-- |
70.8 |
-- |
66.2 |
-- |
63.2 |
-- |
60.4 |
-- |
-- |
-- |
| Cigarettes |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| NSDUH |
33.3 |
31.0 |
29.2 |
26.7 |
25.9 |
23.7 |
23.1 |
22.3 |
20.5 |
19.1 |
17.4 |
15.7 |
14.2 |
13.2 |
11.6 |
10.8 |
9.6 |
9.0 |
7.2 |
| MTF |
39.4 |
35.7 |
34.3 |
32.4 |
30.4 |
28.4 |
26.1 |
26.4 |
26.5 |
24.4 |
21.6 |
20.3 |
18.1 |
16.6 |
13.7 |
12.7 |
12.6 |
12.1 |
12.7 |
| YRBS |
-- |
58.4 |
-- |
54.3 |
-- |
50.3 |
-- |
46.3 |
-- |
44.7 |
-- |
41.1 |
-- |
32.3 |
-- |
28.9 |
-- |
24.1 |
-- |
LSD = lysergic acid diethylamide; MTF = Monitoring the Future; NSDUH = National Survey on Drug Use and Health; YRBS = Youth Risk Behavior Survey.
* Low precision; estimate not reported.
-- Not available.
NOTE: NSDUH data are for youths aged 12 to 17. Some 2006 to 2010 NSDUH estimates may differ from previously published estimates due to updates (see Chapter 3 of this report). Estimates in the 2020 column are italicized to indicate caution should be used when comparing estimates between 2020 and prior years because of methodological changes for 2020. Due to these changes, significance testing between 2020 and prior years was not performed. See Chapters 2, 3, and 6 of this report for more details.
NOTE: MTF data are simple averages of estimates for 8th and 10th graders. MTF data for 8th and 10th graders are reported in Miech et al. (2021). This table does not present any significance testing results. However, testing results may be reported in Miech et al. (2021). MTF data collection for 2020 was halted in schools on March 15, 2020, because of the coronavirus disease 2019 (COVID-19) pandemic. The smaller 2020 sample did not differ from nationally representative results from previous years in terms of demographic characteristics and substance use prevalence estimates that had been stable in recent years.
NOTE: YRBS data are available only every other year. No new 2020 estimates are available, and no significance testing has been performed.
Sources: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2002-2019, and Quarters 1 and 4, 2020; National Institute on Drug Abuse, Monitoring the Future Study, University of Michigan, 2002-2020; Centers for Disease Control and Prevention, Youth Risk Behavior Survey, 2003, 2005, 2007, 2009, 2011, 2013, 2015, 2017, and 2019. |
Table 5.2 – NSDUH, MTF, and YRBS Past Year Prevalence Estimates: Among Youths; Percentages, 2002-2020
Substance/
Survey |
2002 |
2003 |
2004 |
2005 |
2006 |
2007 |
2008 |
2009 |
2010 |
2011 |
2012 |
2013 |
2014 |
2015 |
2016 |
2017 |
2018 |
2019 |
2020 |
| Marijuana |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| NSDUH |
15.8 |
15.0 |
14.5 |
13.3 |
13.2 |
12.5 |
13.1 |
13.7 |
14.0 |
14.2 |
13.5 |
13.4 |
13.1 |
12.6 |
12.0 |
12.4 |
12.5 |
13.2 |
10.1 |
| MTF |
22.5 |
20.5 |
19.7 |
19.4 |
18.5 |
17.5 |
17.4 |
19.3 |
20.6 |
20.7 |
19.7 |
21.3 |
19.5 |
18.6 |
16.7 |
17.8 |
19.0 |
20.3 |
19.7 |
| YRBS |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
| Cocaine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| NSDUH |
2.1 |
1.8 |
1.6 |
1.7 |
1.6 |
1.5 |
1.2 |
1.0 |
1.0 |
0.9 |
0.7 |
0.5 |
0.7 |
0.6 |
0.5 |
0.5 |
0.4 |
0.4 |
0.3 |
| MTF |
3.2 |
2.8 |
2.9 |
2.9 |
2.6 |
2.7 |
2.4 |
2.2 |
1.9 |
1.7 |
1.6 |
1.5 |
1.3 |
1.4 |
1.1 |
1.1 |
1.2 |
1.1 |
0.8 |
| YRBS |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
| Heroin |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| NSDUH |
0.2 |
0.1 |
0.2 |
0.1 |
0.1 |
0.1 |
0.2 |
0.1 |
0.1 |
0.2 |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
0.0 |
* |
* |
| MTF |
1.0 |
0.8 |
1.0 |
0.9 |
0.9 |
0.8 |
0.9 |
0.8 |
0.8 |
0.8 |
0.6 |
0.6 |
0.5 |
0.4 |
0.3 |
0.3 |
0.3 |
0.3 |
0.2 |
| YRBS |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
| LSD |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| NSDUH |
1.3 |
0.6 |
0.6 |
0.6 |
0.4 |
0.5 |
0.7 |
0.6 |
0.6 |
0.6 |
0.6 |
0.6 |
0.9 |
1.0 |
0.8 |
1.0 |
0.8 |
1.1 |
0.9 |
| MTF |
2.1 |
1.5 |
1.4 |
1.4 |
1.3 |
1.5 |
1.6 |
1.5 |
1.6 |
1.5 |
1.3 |
1.4 |
1.3 |
1.5 |
1.5 |
1.5 |
1.5 |
1.6 |
1.8 |
| YRBS |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
| Alcohol |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| NSDUH |
34.6 |
34.3 |
33.9 |
33.3 |
33.0 |
31.9 |
31.0 |
30.5 |
28.7 |
27.8 |
26.3 |
24.6 |
24.0 |
22.7 |
21.6 |
21.9 |
20.8 |
21.2 |
18.5 |
| MTF |
49.4 |
48.3 |
47.5 |
45.3 |
44.7 |
44.1 |
42.3 |
41.6 |
40.7 |
38.4 |
36.1 |
34.6 |
32.4 |
31.5 |
28.0 |
28.0 |
28.3 |
28.5 |
30.6 |
| YRBS |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
| Cigarettes |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| NSDUH |
20.3 |
19.0 |
18.4 |
17.3 |
17.0 |
15.7 |
15.1 |
15.1 |
14.2 |
13.2 |
11.8 |
10.3 |
8.9 |
8.1 |
7.2 |
6.3 |
5.5 |
5.4 |
3.9 |
| MTF |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
| YRBS |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
LSD = lysergic acid diethylamide; MTF = Monitoring the Future; NSDUH = National Survey on Drug Use and Health; YRBS = Youth Risk Behavior Survey.
* Low precision; estimate not reported.
-- Not available.
NOTE: NSDUH data are for youths aged 12 to 17. Some 2006 to 2010 NSDUH estimates may differ from previously published estimates due to updates (see Chapter 3 of this report). Estimates in the 2020 column are italicized to indicate caution should be used when comparing estimates between 2020 and prior years because of methodological changes for 2020. Due to these changes, significance testing between 2020 and prior years was not performed. See Chapters 2, 3, and 6 of this report for more details.
NOTE: MTF data are simple averages of estimates for 8th and 10th graders. MTF data for 8th and 10th graders are reported in Miech et al. (2021). This table does not present any significance testing results. However, testing results may be reported in Miech et al. (2021). MTF data collection for 2020 was halted in schools on March 15, 2020, because of the coronavirus disease 2019 (COVID-19) pandemic. The smaller 2020 sample did not differ from nationally representative results from previous years in terms of demographic characteristics and substance use prevalence estimates that had been stable in recent years.
NOTE: YRBS data are available only every other year. No new 2020 estimates are available, and no significance testing has been performed.
Sources: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2002-2019, and Quarters 1 and 4, 2020; National Institute on Drug Abuse, Monitoring the Future Study, University of Michigan, 2002-2020; Centers for Disease Control and Prevention, Youth Risk Behavior Survey, 2003, 2005, 2007, 2009, 2011, 2013, 2015, 2017, and 2019. |
Table 5.3 – NSDUH, MTF, and YRBS Past Month Prevalence Estimates: Among Youths; Percentages, 2002-2020
Substance/
Survey |
2002 |
2003 |
2004 |
2005 |
2006 |
2007 |
2008 |
2009 |
2010 |
2011 |
2012 |
2013 |
2014 |
2015 |
2016 |
2017 |
2018 |
2019 |
2020 |
| Marijuana |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| NSDUH |
8.2 |
7.9 |
7.6 |
6.8 |
6.7 |
6.7 |
6.7 |
7.4 |
7.4 |
7.9 |
7.2 |
7.1 |
7.4 |
7.0 |
6.5 |
6.5 |
6.7 |
7.4 |
5.9 |
| MTF |
13.1 |
12.3 |
11.2 |
10.9 |
10.4 |
10.0 |
9.8 |
11.2 |
12.4 |
12.4 |
11.8 |
12.5 |
11.6 |
10.7 |
9.7 |
10.6 |
11.2 |
12.5 |
11.6 |
| YRBS |
-- |
22.4 |
-- |
20.2 |
-- |
19.7 |
-- |
20.8 |
-- |
23.1 |
-- |
23.4 |
-- |
21.7 |
-- |
19.8 |
-- |
21.7 |
-- |
| Cocaine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| NSDUH |
0.6 |
0.6 |
0.5 |
0.6 |
0.4 |
0.4 |
0.4 |
0.3 |
0.2 |
0.3 |
0.1 |
0.2 |
0.2 |
0.2 |
0.1 |
0.1 |
0.0 |
0.1 |
0.0 |
| MTF |
1.4 |
1.1 |
1.3 |
1.3 |
1.3 |
1.1 |
1.0 |
0.9 |
0.8 |
0.8 |
0.7 |
0.7 |
0.6 |
0.7 |
0.4 |
0.5 |
0.5 |
0.5 |
0.3 |
| YRBS |
-- |
-- |
-- |
3.4 |
-- |
3.3 |
-- |
2.8 |
-- |
3.0 |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
| Heroin |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| NSDUH |
0.0 |
0.1 |
0.1 |
0.1 |
0.1 |
0.0 |
0.1 |
0.1 |
0.0 |
0.1 |
* |
0.1 |
0.1 |
0.0 |
0.0 |
0.0 |
0.0 |
* |
* |
| MTF |
0.5 |
0.4 |
0.5 |
0.5 |
0.4 |
0.4 |
0.4 |
0.4 |
0.4 |
0.4 |
0.3 |
0.3 |
0.4 |
0.2 |
0.2 |
0.2 |
0.1 |
0.2 |
0.2 |
| YRBS |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
| LSD |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| NSDUH |
0.2 |
0.2 |
0.2 |
0.1 |
0.1 |
0.1 |
0.2 |
0.1 |
0.2 |
0.1 |
0.1 |
0.2 |
0.3 |
0.2 |
0.2 |
0.2 |
0.2 |
0.3 |
0.1 |
| MTF |
0.7 |
0.6 |
0.6 |
0.6 |
0.6 |
0.6 |
0.6 |
0.5 |
0.7 |
0.6 |
0.4 |
0.6 |
0.5 |
0.5 |
0.6 |
0.6 |
0.5 |
0.8 |
0.8 |
| YRBS |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
| Alcohol |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| NSDUH |
17.6 |
17.7 |
17.6 |
16.5 |
16.7 |
16.0 |
14.7 |
14.8 |
13.6 |
13.3 |
12.9 |
11.6 |
11.5 |
9.6 |
9.2 |
9.9 |
9.0 |
9.4 |
8.2 |
| MTF |
27.5 |
27.6 |
26.9 |
25.2 |
25.5 |
24.7 |
22.4 |
22.7 |
21.4 |
20.0 |
19.3 |
18.0 |
16.3 |
15.6 |
13.6 |
13.9 |
13.4 |
13.2 |
15.1 |
| YRBS |
-- |
44.9 |
-- |
43.3 |
-- |
44.7 |
-- |
41.8 |
-- |
38.7 |
-- |
34.9 |
-- |
32.8 |
-- |
29.8 |
-- |
29.2 |
-- |
| Cigarettes |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| NSDUH |
13.0 |
12.2 |
11.9 |
10.8 |
10.4 |
9.9 |
9.2 |
9.0 |
8.4 |
7.8 |
6.6 |
5.6 |
4.9 |
4.2 |
3.4 |
3.2 |
2.7 |
2.3 |
1.4 |
| MTF |
14.2 |
13.5 |
12.6 |
12.1 |
11.6 |
10.6 |
9.6 |
9.8 |
10.4 |
9.0 |
7.9 |
6.8 |
5.6 |
5.0 |
3.8 |
3.5 |
3.2 |
2.9 |
2.7 |
| YRBS |
-- |
21.9 |
-- |
23.0 |
-- |
20.0 |
-- |
19.5 |
-- |
18.1 |
-- |
15.7 |
-- |
10.8 |
-- |
8.8 |
-- |
6.0 |
-- |
LSD = lysergic acid diethylamide; MTF = Monitoring the Future; NSDUH = National Survey on Drug Use and Health; YRBS = Youth Risk Behavior Survey.
* Low precision; estimate not reported.
-- Not available.
NOTE: NSDUH data are for youths aged 12 to 17. Some 2006 to 2010 NSDUH estimates may differ from previously published estimates due to updates (see Chapter 3 of this report). Estimates in the 2020 column are italicized to indicate caution should be used when comparing estimates between 2020 and prior years because of methodological changes for 2020. Due to these changes, significance testing between 2020 and prior years was not performed. See Chapters 2, 3, and 6 of this report for more details.
NOTE: MTF data are simple averages of estimates for 8th and 10th graders. MTF data for 8th and 10th graders are reported in Miech et al. (2021). This table does not present any significance testing results. However, testing results may be reported in Miech et al. (2021). MTF data collection for 2020 was halted in schools on March 15, 2020, because of the coronavirus disease 2019 (COVID-19) pandemic. The smaller 2020 sample did not differ from nationally representative results from previous years in terms of demographic characteristics and substance use prevalence estimates that had been stable in recent years.
NOTE: YRBS data are available only every other year. No new 2020 estimates are available, and no significance testing has been performed.
Sources: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2002-2019, and Quarters 1 and 4, 2020; National Institute on Drug Abuse, Monitoring the Future Study, University of Michigan, 2002-2020; Centers for Disease Control and Prevention, Youth Risk Behavior Survey, 2003, 2005, 2007, 2009, 2011, 2013, 2015, 2017, and 2019. |
6. Special Methodological Issues for the 2020 NSDUH
Data collection on the 2020 National Survey on Drug Use and Health (NSDUH) commenced in early January 2020 according to procedures in prior survey years. On January 31, 2020, U.S. Department of Health and Human Services Secretary Alex M. Azar II declared a public health emergency in the United States to aid in the response to coronavirus disease 2019 (COVID-19) (U.S. Department of Health and Human Services, 2020). As indicated elsewhere in this report, the Substance Abuse and Mental Health Services Administration (SAMHSA) suspended in-person data collection on the 2020 NSDUH on March 16, 2020, because of the COVID-19 pandemic. This chapter discusses special methodological issues for the 2020 NSDUH because of the COVID-19 pandemic and implications for 2020 NSDUH estimates.
6.1 Background on the 2020 NSDUH and the COVID-19 Pandemic
This section provides a general overview of methodological changes for the 2020 NSDUH because of the COVID-19 pandemic. This section also discusses hypothesized and initial findings on how behavioral health outcomes in the United States changed during the COVID-19 pandemic and limitations in measuring these changes. The section concludes with an overview of the challenges in measuring the independent effects of methodological changes in the 2020 NSDUH.
6.1.1 Overview of Methodological Changes for the 2020 NSDUH
SAMHSA’s decision in mid-March 2020 to suspend in-person data collection on the 2020 NSDUH was consistent with decisions among other national surveys that collect data from respondents in person. Options that national surveys considered for resuming data collection included (1) stopping indefinitely and waiting to resume data collection when in-person contact was once again feasible, (2) stopping temporarily to redesign some aspect of the data collection and resuming with a different design, or (3) continuing to collect data with an evolving design that excluded in-person data collection.
SAMHSA determined that the second option was the best course of action for NSDUH. The plan for resuming 2020 data collection on NSDUH involved adding a web survey component and resuming in-person data collection (where feasible) in Quarter 4. In-person interviewing for Quarter 4 began on October 1, 2020, and web-based data collection began on October 30, 2020. The data collection period ran through December 31, 2020. The main goal for this change in data collection procedures was to complete enough Quarter 4 interviews to produce 2020 national estimates for the civilian, noninstitutionalized population aged 12 or older, while meeting precision requirements and accounting for effects of the methodological changes. One consequence of NSDUH choosing the second option was that there was a gap in full-scale data collection between Quarter 1 and Quarter 4.
Because the plan for 2020 Quarter 4 was to conduct in-person interviewing in all segments where feasible, SAMHSA authorized RTI to conduct a small-scale in-person data collection for 1 week in July 2020. This small-scale data collection effort is described in Section 2.2.1.1.2. This small-scale data collection was successful in informing safe field procedures for resuming in-person data collection in Quarter 4.
Based on the experience from the 1 week of in-person data collection in July 2020, plans for Quarter 4 included adding a web survey component for sample dwelling units (SDUs) in two situations: (1) SDUs could be assigned to the web mode according to COVID-19 infection metrics at the start of Quarter 4; and (2) in areas where SDUs were initially selected for in-person data collection but became ineligible because of increases in COVID-19 infection metrics, web transition letters were sent to SDUs where household screening or interviewing was not complete. In this second situation, the web transition letters included instructions on how to complete the screening and interview via the web.
Because of the high number of areas in which SDUs were ineligible for in-person interviewing during Quarter 4, 19,202 (93.0 percent) of the 20,656 interviews completed in Quarter 4 were completed via the web (see Table 3.7). One implication of most of the Quarter 4 interviews being completed via the web was that the screening response rate (SRR) in Quarter 4 was lower than the SRR for in-person screenings in Quarter 1 (see Section 3.3.1.1). This result was expected, given the lower response rates for web surveys compared with in-person surveys and the few national household surveys in the United States currently using the web for household screening (Daikeler et al., 2020).
The total number of interviews completed in Quarter 4 surpassed the minimum number of interviews needed to produce national estimates with acceptable precision for people aged 12 or older. However, the number of interviews from adolescents aged 12 to 17 in Quarter 4 was lower than expected from the size of the sample that was released. Unweighted data indicate that among the 20,656 interviews in Quarter 4, 2,401 were from adolescents, or 11.6 percent of all Quarter 4 interviews. In comparison, 3,936 of the 15,628 interviews in Quarter 1 (which were conducted in person) were from adolescents, or 25.2 percent of all Quarter 1 interviews.
A likely explanation for the reduced number of completed interviews from adolescents in Quarter 4 is that the process of obtaining parental permission and youth assent required additional steps for web-based data collection to ensure that adults who provided permission for youths to participate via the web were the actual parents of the sampled youths (see Section 2.2.1.3). For in-person data collection, FIs could obtain parental permission without the selected youth(s) also needing to be present.
A final difference between Quarter 1 and Quarter 4 was that new questions relevant to the COVID-19 pandemic were added to the Quarter 4 interviews. These new questions could have created context effects (see Section 3.4.6) for subsequent questions in the Quarter 4 interview. Such context effects would not be present for those questions in data from Quarter 1 or earlier years (in the absence of other questionnaire changes before Quarter 4).
6.1.2 Hypothesized and Initial Findings for the COVID-19 Pandemic and Behavioral Health Outcomes
6.1.2.1 Potential Popular Expectations
News stories in 2020 could create popular expectations that the COVID-19 pandemic negatively affected substance use and mental health outcomes in the United States, despite the stories not presenting population estimates from national surveys. For example, The Washington Post reported that sales of alcoholic beverages spiked 55 percent in the week ending on March 21 (Reiley, 2020), the first week for which many states issued stay-at-home orders due to the COVID-19 pandemic. The article also noted online sales of alcohol in the United States rose almost five-fold in April 2020 compared with the same period in 2019. However, retail sales data could be misleading if they captured information from retail outlets that were allowed to stay open during the COVID-19 pandemic as “essential” businesses, but they did not capture decreases in on-premise alcohol consumption in bars or restaurants that were closed (Nurin, 2020).
6.1.2.2 Initial Investigations and Findings
As the COVID-19 pandemic continued, federal agencies and other organizations repeated surveys with questions asked in prior implementations, added new questions related to COVID-19 to existing surveys, or devised new surveys to measure the potential impact of the COVID-19 pandemic on substance use or mental health. Given the restrictions on in-person contact, nearly all surveys used web-based data collection to measure the impact of the COVID-19 pandemic. SAMHSA summarized preliminary research available through November 30, 2020, in a Disaster Technical Assistance Center Supplemental Research Bulletin titled A Preliminary Look at the Mental Health and Substance Use-related Effects of the COVID-19 Pandemic (SAMHSA, 2021).
The bulletin cited findings from three surveys that found increases in alcohol use during 2020:
- Barbosa et al. (2020) fielded a cross-sectional online survey in May 2020 of 993 individuals using a probability-based panel designed to be representative of the U.S. population aged 21 and older.
- Respondents reported consuming 29 percent more drinks per day in April 2020 than in February 2020.
- Compared with reports in February 2020, reports in April 2020 also were 20 percent higher for adults exceeding recommended drinking limits and were 21 percent higher for binge drinking.
- Grossman et al. (2020) fielded a cross-sectional online survey in May 2020 with a convenience sample of adults aged 21 or older in the United States.
- About 34 percent of participants reported binge drinking, and 7 percent reported extreme binge drinking.
- Reasons reported for increased drinking included increased stress (46 percent), increased alcohol availability (34 percent), and boredom (30 percent).
- Pollard et al. (2020) collected data using the RAND Corporation American Life Panel, a nationally representative, probability-sampled panel of 6,000 participants aged 18 or older who speak English or Spanish.
- Compared with a baseline survey conducted from April 29 through June 9, 2019, the frequency of alcohol consumption significantly increased overall by 14 percent.
- Significant increases compared with the 2019 baseline were observed for women, adults aged 30 to 59, and non-Hispanic White individuals.
In addition, Czeisler and colleagues (2020) conducted a web survey in June 2020 of 5,412 adults in the United States. About 13 percent of respondents reported they had begun or increased substance use to cope with stress or emotions associated with the COVID-19 pandemic.
Data from Europe indicated some temporary decreases in substance use during the COVID-19 pandemic. Initial lockdowns appeared to lead to a decrease in consumption of some illicit drugs due to disruptions in drug markets. These reductions began to disappear as social distancing measures were eased (European Monitoring Centre for Drugs and Drug Addiction, 2021).
For assessing the potential impact of the COVID-19 pandemic on mental health, new and repeated surveys provided relevant data:
- As Section 5.1.3 details, the U.S. Census Bureau, in collaboration with multiple federal agencies, initiated the Household Pulse Survey in spring 2020.
- Occurrences of symptoms of depressive disorder ranged from about 22 percent for the period from April 28 to May 10, 2020, to about 30 percent from December 9 to 21, 2020.
- Occurrences of symptoms of anxiety disorder over this period ranged from about 26 percent for the period from April 28 to May 10, 2020, to about 37 percent from November 11 to 23, 2020.140
- The American Psychological Association (2020) conducts an annual national survey on stress and mental health in the United States.
- The 2020 survey was administered in August and included 3,409 adult and 1,026 youth participants aged 13 to 17.
- Among the participants in 2020, 19 percent described their mental health as worse than at the same time in 2019.
In addition, more recent findings suggest that levels of anxiety, depression, and broader psychological distress among adults increased during early stages of the COVID-19 pandemic in March and April 2020 but that levels of psychological distress declined by mid-June 2020 (Aknin et al., 2021; Daly & Robinson, 2021). The hypothesis was that acute symptoms of psychological distress subsided as adults learned to cope with changes during the COVID-19 pandemic and adjust their lifestyle accordingly. Stated another way, people could have been more resilient in coping with the challenges of the COVID-19 pandemic than initial impressions might have suggested from the early stages of the COVID-19 pandemic, although people (especially those with pre-existing mental health issues) still could have needed access to mental health services.
6.1.3 Data Limitations in Measuring Changes during the COVID-19 Pandemic
Findings noted in Section 6.1.2 need to be interpreted with caution for multiple reasons. For example, Czeisler and colleagues (2020) asked whether respondents started using substances or increased their use but did not ask whether respondents stopped or reduced their substance use during the COVID-19 pandemic. Therefore, increases in substance use during the COVID-19 pandemic could be offset in part by people who decreased their use. In addition, reports of new or increased substance use do not readily translate to changes in prevalence.
Potential limitations of the web survey data collected in 2020 must also be considered. A first reason for caution is that these web surveys used different samples, methods, and questions or scales in collecting data. Differences in substance use behavior and mental health estimates observed among these studies and differences between any of these surveys and NSDUH are subject to varying effects of survey errors. The main types of survey errors that can affect all surveys include noncoverage bias, nonresponse bias, and measurement errors. Differences in data collection periods, modes employed, response rates, measures used, and other aspects of the survey design could lead to different estimates. One further consideration is that most new data collection efforts fielded in 2020 did not have pre-COVID-19 pandemic data using the same methods for comparison.141
Consequently, comparison of mental health outcomes from web surveys conducted in 2020 with national surveys can be misleading. For example, Czeisler and colleagues (2020) used an online market research panel and quota sampling. In the Household Pulse Survey, response rates ranged from 1.3 to 3.8 percent in the first 3 weeks of data collection from April to May 2020. In comparison, the 2019 National Health Interview Survey (NHIS) had a household response rate of 61.1 percent and a final sample adult response rate of 59.1 percent (National Center for Health Statistics [NCHS], 2020).
A second reason for caution in interpreting data collected in 2020 is that the COVID-19 pandemic in the United States has not been a unitary event. Data from the Centers for Disease Control and Prevention (CDC) showed three peaks in the number of new COVID-19 cases in the United States in 2020: (1) in March and April, (2) in July, and (3) and in late November and December (CDC, n.d.). Similarly, policy measures to contain the spread of the virus or mitigate its effects varied over time, as did people’s responses to these policies. For example, the National Institute on Alcohol Abuse and Alcoholism’s Alcohol Policy Information System indicated that as of April 15, 2020, bars and restaurants were closed in all 50 states except for South Dakota. As of June 15, 2020, no states ordered restaurants to be closed, and some states allowed all restaurants to be open with no apparent restrictions. Regulations fluctuated in subsequent periods (National Institute on Alcohol Abuse and Alcoholism, 2021). Therefore, studies that had a relatively narrow period of data collection period in 2020 could provide a limited “snapshot” of the effects of the COVID-19 pandemic on substance use and mental health.
A third reason for caution is that few surveys included a design or analytic methods to establish a clear link between the COVID-19 pandemic and substance use behaviors or mental health conditions. For NSDUH, changes in substance use or mental health outcomes during the COVID-19 pandemic are confounded by methodological changes in the 2020 survey (Sections 3.3.3 and 6.1.4). As discussed in Section 5.1.7, the 2020 NHIS included four separate designs in response to the COVID-19 pandemic. As for the 2020 NSDUH, the task at hand for the NHIS is determining how to use weighting and estimation techniques to produce official 2020 estimates from the disparate pieces, each of which has its own coverage and nonresponse issues (NCHS, 2021).
A fourth and final caution is that the COVID-19 pandemic was not the only major event in 2020 that could have affected substance use behavior and mental health conditions. Shifts in recent trends in substance use and mental health in 2020 could have resulted from the impact of other events or the impact of COVID-19 and other events combined. For example, protests broke out in cities across the United States in late spring and summer 2020 following the death of George Floyd on May 25, 2020, in Minneapolis police custody (Taylor, 2021). The Household Pulse Survey also reported a heightened level of symptoms of anxiety during the period from October 28 to November 23, 2020, which led up to and immediately followed the 2020 Presidential election in the United States (NCHS, n.d.-a). Survey research cannot control for the effects of a health pandemic that is occurring simultaneously with other major types of social disruptions.
6.1.4 Challenges in Assessing the Impact of Methodological Changes in the 2020 NSDUH
As noted in Section 6.1.1, 2020 data were collected completely in person for Quarter 1, but Quarter 4 data were collected using either in-person or web modes. Moreover, only limited NSDUH data were collected in the middle two quarters of 2020, from April to September. Section 6.1.2 also indicated that use of some substances (especially alcohol) and mental health conditions (especially anxiety and depression) could have been increasing in 2020 during different stages of the COVID-19 pandemic in the United States. These two coinciding factors created substantial challenges for assessing the effects of methodological changes, such as having only limited data in Quarters 2 and 3 and adding web-based data collection in Quarter 4.
Assignment of segments to modes (i.e., in person or web) in Quarter 4 was dictated by whether COVID-19 infection metrics made the segments eligible for in-person interviewing. Randomly assigning segments or SDUs to receive different data collection modes was not ethical in the midst of a pandemic. Relatively few segments in Quarter 4 were eligible for in-person data collection, and eligibility for in-person data collection decreased during the quarter. Moreover, the characteristics of segments where data were collected in person differed between Quarters 1 and 4. Consequently, comparisons limited to in-person data from Quarters 1 and 4 control for survey mode but would not be valid for assessing changes in substance use or mental health from early to late 2020. The availability of only limited data in Quarters 2 and 3 due to COVID-19 further limited observation of trends in substance use and mental health over the course of the year. The availability of more extensive NSDUH data from the middle of 2020 would have presented a more complete picture of substance use and mental health in the United States in 2020 during the COVID-19 pandemic.
Second, multimode data collection could have introduced effects on estimates that are difficult to disentangle from actual changes in substance use behavior or mental health conditions over the course of 2020. For example, changes in response rates because of different data collection modes could increase the potential for nonresponse bias in key estimates for 2020; changes in nonresponse bias for 2020 could obscure or accentuate differences in estimates between 2020 and those in prior years. As noted in Section 3.3.1.1, the weighted overall response rate (ORR) for Quarter 1 was 42.9 percent, and the weighted ORR for Quarter 4 was 6.6 percent. The weighted interview response rate (IRR) in Quarter 1 was 63.2 percent, and the weighted IRR in Quarter 4 was 59.5 percent. The SRR in Quarter 4, when data were collected predominantly by web, was the greater factor in the effect on the Quarter 4 ORR.
One way to disentangle the effects of methodological differences and patterns in substance use and mental health during the COVID-19 pandemic would be to implement a controlled experiment in future quarters of data collection. Such an experiment could help to identify the independent effects of methodological differences and the COVID-19 pandemic on estimates. The experimental design would randomize data collection modes across segments when COVID-19 infection rates are much lower and when an increased percentage of the population has been fully vaccinated. This design would allow for establishing clear trends in substance use behavior and mental health conditions at the start of data collection to compare with the experimental results from both modes. Analysis of the experiment could then identify significant differences observed between key substance use and mental health estimates that are specifically attributable to mode effects such as nonresponse bias and effects on reporting.
6.2 Effects of Methodological Changes on Data Processing and Estimation
6.2.1 Effects on Imputation
In response to the NSDUH methodological changes described previously that were introduced during 2020 in response to the COVID-19 pandemic, imputation procedures were modified for 2020, as described in Section 2.3.3.2. Changes to the data collection period and methodology in the 2020 NSDUH also necessitated changes to the imputation procedures for 2020. This section focuses on the reasons for the changes to the imputation procedures.
6.2.1.1 Anticipated Effects on Imputation
Anticipated effects of changes to data collection on the 2020 data informed changes to the imputation procedures described as follows:
- Differences in data collection mode: As noted in Section 6.1.1, all Quarter 1 interviews were administered in person. COVID-19 infection rate metrics determined the data collection mode (in person or web) that SDUs received in Quarter 4.
- Consequently, the interview mode was expected to be correlated with outcome measures that are imputed for NSDUH (e.g., recency and frequency of substance use, past year substance use disorder [SUD]).
- These correlations could occur because of differences in COVID-19 infection rates during data collection that directly affected imputed outcomes or through differences in demographic and socioeconomic characteristics between in-person and web respondents that could affect other outcomes.
- Differences in item nonresponse (i.e., missing data): Item response rates were hypothesized to differ by mode of data collection.
- Items for imputed variables that were administered by FIs during in-person data collection were self-administered for web administration (e.g., race and Hispanic origin, health insurance coverage, income).
- Web survey respondents were also hypothesized to be more likely than in-person respondents to break off the interview without completing it because FIs were not present to keep web respondents engaged.
- Consequently, variables that undergo imputation were expected to require imputation for a higher percentage of web survey respondents than for in-person respondents.
- Quarterly differences due to the COVID-19 pandemic: Seasonality and differences in infection rates, policies, and responses to policies during different stages of the COVID-19 pandemic in the United States could affect outcomes.
- Substance use patterns such as recency of use, frequency of use, and past year initiation of misuse could differ for respondents interviewed in Quarter 1 versus those interviewed in Quarter 4, particularly for recall periods such as use in the past month or past year.
- Similarly, respondents in Quarter 1 could differ from respondents in Quarter 4 for measures such as current employment status, health insurance coverage, and household composition because of COVID-19.
- Therefore, combining the data from Quarters 1 and 4 to fit prediction models and select final donors for imputation (e.g., imputing Quarter 1 data from a Quarter 4 donor or vice versa) could attenuate actual differences in response patterns across quarters.
To allow time for any changes to the imputation procedures to be implemented without negatively affecting the timeline for the release of publications from the 2020 NSDUH, decisions about modifications to the imputation procedures had to be made prior to the conclusion of data collection for 2020. Further, the data collection mode in Quarter 4 was determined by COVID-19 infection rates instead of by selection probabilities as part of a designed experiment. Consequently, it was not possible to disentangle the effects of the various methodological changes to verify some of the assumptions guiding these a priori decisions for imputation.
Given the anticipated effects described previously and available information, however, the imputation procedures for the 2020 NSDUH were modified as follows:
- Quarter 1 and Quarter 4 data were imputed separately to incorporate information about the quarter of data collection.
- Interview mode was included as a covariate in the imputation models for Quarter 4 to take into account anticipated effects on data because of interview mode.
6.2.1.2 Observed Outcomes for Imputation
An analysis conducted after data collection supported many of the expectations about the effects of the methodological changes on the 2020 NSDUH data. As seen in Tables 2.3 and 2.4 in Chapter 2, respondents in Quarter 4 (when interviews were predominately completed via the web) generally required imputation at a higher rate than for Quarter 1 respondents (when interviews were exclusively conducted in person).
These differences in imputation rates were particularly large for variables from later sections of the interview that are interviewer administered during in-person data collection (i.e., household composition, health insurance coverage, and income). The higher imputation rates in these sections are largely attributable to two factors:
- For questions that were interviewer administered for in-person respondents, a higher percentage of web respondents in Quarter 4 answered corresponding questions as “don’t know” or “refused” compared with in-person respondents from all quarters (Table 6.1).
- An average of 1.36 percent of in-person respondents from all quarters answered interviewer-administered questions as “don’t know” or “refused” for questions that were received by at least 100 respondents.
- An average of 2.17 percent of web respondents in Quarter 4 answered questions as “don’t know” or “refused” for these same questions that were administered to at least 100 web respondents. These questions were self-administered for web respondents.
- A substantial contribution to higher imputation rates among web respondents was from respondents who met usability criteria (Section 2.3.1) but broke off the interview before completing it (Table 6.2).
- Among all Quarter 4 respondents, the mean imputation rate was 11.69 percent for the six variables from the health insurance section of the questionnaire.
- The mean imputation rate dropped to 1.67 percent when data were excluded from the Quarter 4 respondents who ended the interview prior to the health insurance section.
- When break-offs were excluded, mean imputation rates also dropped in the SUD and emerging issues sections that are self-administered for in-person data collection (and self-administered for web respondents).
In addition to differences in rates of imputation, variations in substance use patterns and the demographic and socioeconomic characteristics of respondents were observed between Quarters 1 and 4. Imputing the data separately by quarter helped to ensure that these differences were reflected in the imputed data through the imputation model coefficients and selection of donors:
- Raw estimates for alcohol and tobacco use in the past month and past year periods (i.e., prior to editing or imputation of data described in Sections 2.3.2 and 2.3.3) were lower in Quarter 4 than Quarter 1. However, raw estimates of methamphetamine use in the past month were higher in Quarter 4 (see Table 6.3).
- Respondents in Quarter 4 also had lower rates of employment relative to respondents in Quarter 1 (see Table 6.4).
6.2.2 Effects on Person-Level Weighting
Weighting procedures were modified for the 2020 NSDUH to account for the disruption in data collection because of the COVID-19 pandemic (i.e., no data collection in Quarter 2 and extremely limited data collection in Quarter 3), to account for differences in data collection modes in Quarters 1 and 4 (i.e., exclusively in-person data collection in Quarter 1 and predominantly web-based data collection in Quarter 4), and to accommodate analytic needs. This section focuses on the reasons for the modifications to the weighting procedures that were described in Section 2.3.4. To recap briefly, key modifications were made to the weighting procedures for the 2020 NSDUH:
- Separate person-level analysis weights were developed for Quarters 1 and 4.
- An overall analysis weight was devised for the combined data from Quarters 1 and 4 using a modified approach.
- Educational attainment was included in the last poststratification adjustment for Quarters 1 and 4.
- Additional person-level analysis weights were developed to take into account missing data because of web break-offs.
6.2.2.1 Development of Quarterly and Combined 2020 Analysis Weights
This section first discusses reasons for developing separate quarterly analysis weights for the 2020 NSDUH. Discussion of the reasons is followed by a discussion of the creation of overall analysis weights for the combined data from Quarters 1 and 4.
6.2.2.1.1 Reasons for the Development of Quarterly Weights
Reasons for developing separate person-level analysis weights for Quarters 1 and 4 are described as follows:
- Temporal differences in 2020 during the COVID-19 pandemic: As discussed in Section 6.1.3, there were three peaks in COVID-19 infections in the United States in 2020 (CDC, n.d.).
- Data collection in Quarter 1 occurred in the early stages of the COVID-19 pandemic in the United States, before in-person data collection was suspended.
- A major peak in new COVID-19 cases occurred when Quarter 4 data collection was in progress for NSDUH.
- In addition to temporal changes in COVID-19 cases, there were differences in data collection modes between Quarters 1 and 4 for NSDUH.
- Temporal changes in COVID-19 infections, policies to contain the virus, public behavior in response to the virus or policies, and differences in data collection modes by quarter could have affected key outcomes for NSDUH.
- Creation of separate analysis weights for Quarters 1 and 4 would allow population estimates to be produced separately by quarter for analysis.
- Differences in unit nonresponse: SRRs and IRRs differed by quarter.
- As discussed in Section 6.1.1 and shown in Table 3.3, the SRR in Quarter 4 (11.13 percent) was considerably lower than the SRR in Quarter 1 (67.76 percent).
- The IRR in Quarter 4 (59.48 percent) was slightly lower than the IRR in Quarter 1 (63.24 percent), as shown in Tables 3.5 and 3.6, respectively.
- If people in SDUs successfully completed the screening process, the difference in IRRs by quarter was not as great as the difference for SRRs.
- However, IRRs differed between Quarters 1 and 4 in some demographic domains.
- Among youths aged 12 to 17, the IRR was 70.53 percent in Quarter 1 and 25.6 percent in Quarter 4.
- Among Hispanics, the IRRs were 63.59 percent in Quarter 1 and 51.2 percent in Quarter 4.
- Thus, performing separate nonresponse adjustments at the screening and interviewing phases for Quarters 1 and 4 would better address quarterly differences in potential bias due to unit nonresponse.
- Questionnaire changes for Quarter 4: New items were added to NSDUH for Quarter 4. Weights specifically for the Quarter 4 sample needed to be developed to produce population estimates from these items.
6.2.2.1.2 Development of Combined 2020 Analysis Weights
A traditional way to calculate overall analysis weights for the combined data from Quarters 1 and 4 would be to perform a poststratification adjustment for the combined data. In this poststratification adjustment, the nonresponse-adjusted weights from Quarters 1 and 4 would be combined and benchmarked to the census population estimates. In the poststratification adjustment models, quarter would be included in the main effects and interactions with state. However, no interactions between quarter and demographic variables were included as in prior NSDUH years. Because weights in demographic domains would not be controlled for each quarter, weight sums in demographic domains between two quarters could differ after the poststratification adjustment. Outcomes in certain demographic domains could be different between Quarters 1 and 4 because of the mode differences in data collection or COVID-19 effects. When weight sums are shifted between two quarters in demographic domains, survey estimates for Quarters 1 and 4 can be affected. Thus, the approach of performing a poststratification for the combined data to create overall analysis weights for Quarters 1 and 4 is not ideal.
However, separate quarterly analysis weights were developed for the reasons mentioned previously. Therefore, a preferable alternate approach to calculate overall analysis weights for combined data from Quarters 1 and 4 for the 2020 NSDUH would be to combine the quarterly analysis weights and divide them by a factor of 2. In this way, each quarter contributes half of the analysis weights to the combined weights. This approach also preserves quarterly weight sums for demographic domains (e.g., age group, gender, race, Hispanic origin) to ensure that the weight sums in the demographic domains are not shifted between quarters.
6.2.2.2 Educational Attainment in Poststratification Adjustment
This section first discusses reasons for adding educational attainment to the poststratification adjustment for the 2020 NSDUH person-level weights. Discussion of the reasons is followed by a discussion of outcomes for educational attainment.
6.2.2.2.1 Reasons for Adding Educational Attainment to the Poststratification Adjustment
Educational attainment for adults aged 18 or older had not been included in the poststratification models for NSDUH person-level weighting in prior years. As shown in Tables 6.5 and 6.6, the unweighted and weighted distributions for categories of educational attainment among adult respondents have been stable for the 2016 to 2019 NSDUHs, and the weighted distributions are close to the distributions of the corresponding year of the American Community Survey (ACS).
However, the web survey component for the 2020 NSDUH in Quarter 4 was hypothesized to yield a sample of adults with a higher level of educational attainment compared with the in-person samples from Quarter 1 or prior years:
- A Pew Research Center survey in early 2021 indicated that 14 percent of adults with a high school education or less reported that they do not use the Internet compared with 2 percent of adults with a college education or higher (Perrin & Atske, 2021).
- NSDUH respondents taking the survey on the web needed to be able to read English or Spanish (Section 2.2.1.3). In-person respondents with limited reading ability could listen on headphones to audio recordings of self-administered questions (Section 2.2.1.1.1).
6.2.2.2.2 Selected Outcomes for Educational Attainment
As noted previously, the unweighted and weighted distributions of the four-level educational attainment measure (less than high school, high school graduate, some college and associate degree, college graduate) have been very stable in NSDUH from 2016 to 2019 Tables 6.5 and 6.6). The weighted distributions have been in line with the distribution of educational attainment among adults from the 2016-2019 ACS.
The distributions for educational attainment among adults in Quarter 1 of 2020 (for in-person data collection) also were similar to the NSDUH distributions from 2016 to 2019 and with the distribution for the 2019 ACS (Tables 6.5 and 6.6). Adults in Quarter 4 (who principally completed the survey via the web), however, were more likely than adults in Quarter 1 to be college graduates and less likely not to have completed high school or to be high school graduates.
These findings confirmed the need to include educational attainment in the poststratification adjustment for 2020 person-level weights. Imbalance in the weighted educational attainment distributions would create bias in estimates. In 2019, for example, 8.0 percent of adults who were college graduates were cigarette smokers in the past month compared with 26.5 percent of adults who had not graduated from high school (Center for Behavioral Health Statistics and Quality [CBHSQ], 2020a). For this example of cigarette smoking, not adjusting the 2020 person-level weights for the higher proportion of adult respondents in Quarter 4 who were college graduates would depress estimates of cigarette smoking in the past month.
To remove this source of bias, weighted distributions for educational attainment in Quarters 1 and 4 were forced to match the distributions for educational attainment from the 2019 ACS (Section 2.3.4.2). The weighted distributions for educational attainment in the combined data from Quarters 1 and 4 also matched the educational attainment distributions from the 2019 ACS.
6.2.2.3 Creation of Break-Off Analysis Weights
This section first discusses reasons for creating additional person-level break-off analysis weights for the 2020 NSDUH. Discussion of the reasons is followed by a discussion of outcomes for break-offs in the 2020 NSDUH.
6.2.2.3.1 Reasons for Creating Break-Off Analysis Weights
In prior NSDUH years (i.e., using in-person data collection), a negligible percentage of respondents met usability criteria (Section 2.3.1) but broke off the interview without completing it. In 2019, for example, 0.03 percent of respondents (unweighted) who provided usable data broke off the interview without completing it (CBHSQ, 2020b).
However, the in-person interview averaged about an hour to complete (Section 2.2.1.1.1). The web interview was modified as needed for self-administration (Section 2.2.2.4), but the questionnaire was not shortened for web administration (Section 6.3). Further, FIs were not present to keep web respondents engaged in completing the survey. Therefore, web respondents could have a greater likelihood of breaking off the interview compared with in-person respondents.
All remaining data were missing from the point where respondents broke off the interview. Unless variables were imputed to remove missing data, levels of missing data and how missing data were handled could introduce bias in estimates (Section 3.3.2). The higher the level of missing data in a variable, the greater the risk that estimates from that variable will be biased.
6.2.2.3.2 Selected Outcomes for Break-Offs in 2020
In Quarter 4 of 2020, 8.6 percent of respondents aged 12 or older (unweighted) met the usability criteria described in Section 2.3.1, but they did not complete the interview. The corresponding unweighted percentage in Quarter 1 was 0.045 percent (Section 2.3.4.2). Among web respondents in Quarter 4 who broke off the interview, the cumulative percentage who broke off exceeded 50 percent after the mental health section for adults.
It may be reasonable to assume that interview break-offs occurred more at random before the mental health section. If so, then excluding respondents from analyses if they had missing values caused by interview break-offs or applying zero imputation (i.e., treating missing values as equivalent to negative outcomes) would be acceptable solutions. Similarly, interview break-offs among adults in the mental health and adult depression sections could occur at random for reasons unrelated to the questionnaire.
However, interview break-offs by the mental health and adult depression sections for adults or by the youth experiences section for adolescents aged 12 to 17 might not be random occurrences.142 Web respondents with mental health conditions could have chosen to discontinue the survey, rather than answer questions about their mental health. As noted in Section 6.2.1, missing data for many variables are statistically imputed, reducing the risk of bias. However, zero imputation is common for variables in the mental health and subsequent sections that can create substantial risk of bias when combined with a high break-off rate.
To overcome the bias issue because of missing values from break-offs, additional analysis weights were created to analyze unimputed outcome variables beginning with the mental health section for adults and subsequent sections. Adults who did not complete the mental health or adult depression sections were excluded from this weighting adjustment. The main analysis weights for remaining adult respondents were adjusted to the main analysis weights for all adult respondents separately in Quarters 1 and 4. The break-off adjustment was not performed for adolescents aged 12 to 17 because few adolescents in Quarters 1 and 4 broke off the interview.
6.2.3 Effects on Presentation of Estimates
The 2020 NSDUH data were collected during three distinct time periods:
- The Quarter 1 sample was collected from January 4, 2020, through March 16, 2020, and yielded 15,628 final interviews, including 699 interviews from the Clinical Validation Study (CVS). See Sections 2.1.2.1 and 2.2.1.2 for more details about the CVS.
- The Quarter 3 small-scale, in-person data collection lasted for 1 week, from July 16 to 22, 2020, to test new COVID-19 safety protocols for in-person interviews. This sample yielded fewer than 100 completed interviews in a limited number of states.
- Quarter 4 in-person data collection began on October 1, 2020, and was suspended in any areas that became ineligible during the quarter based on COVID-19 infection rate metrics. Web-based data collection began on October 30, 2020. The final Quarter 4 sample yielded 20,656 completed interviews, regardless of the data collection mode.
The data across these quarters were combined to generate the 2020 estimates that are shown in 2020 NSDUH reports and tables. The small number of Quarter 3 interviews were grouped with the Quarter 4 data to create the person-level analysis weights. SAMHSA decided to produce estimates for 2020 using the combined data to increase the sample sizes and resulting precision of the estimates. Estimates for 2020 were presented in the tables and reports along with estimates prior to 2020, when available. Unlike prior years, however, Quarters 2 and 3 were largely unrepresented in the 2020 data. This gap in the data is an important caveat for the combined estimates from the 2020 NSDUH. The final combined sample sizes in 2020 also were considerably smaller than the sample sizes in 2019. Consequently, the 2020 standard errors for estimates were generally higher than those in 2019.
Given the differences in data collection periods and other methodological procedures (see Section 6.1.4) between 2020 and prior NSDUH years, SAMHSA decided not to make statistical comparisons between 2020 estimates and those from prior years. Without a controlled experiment in which segments were randomly assigned to in-person or web-based data collection, SAMHSA was not confident that apparent differences (or the absence of differences) between the 2020 estimates and those in prior years could be attributed to underlying changes (or no change) in the population.
To indicate the potential break in comparability of estimates between 2019 and 2020 in tables and reports for the 2020 NSDUH, SAMHSA decided to use special formatting for the 2020 estimates in tables and figures that showed estimates for 2020 and prior years. Tables and reports also included a note to caution data users about making direct comparisons between estimates in 2020 and those from prior years. The note also referred data users to this report for more information on the changes for 2020.
6.2.3.1 Presentation of Data Available Only from Quarter 4
Section 2.2.2.3 described specific changes to the 2020 NSDUH questionnaire for Quarter 4, regardless of data collection mode. Following is a recap of key changes:
- Questions were added to the relevant sections of the interview to measure the use of virtual (telehealth) services in the past 12 months for substance use treatment, medical care, and mental health care.
- The skip logic was changed for questions about suicide plans or attempts in the past 12 months among adults so that all adults were asked these questions, regardless of whether they reported having serious thoughts of suicide in the past 12 months.
- Items were added for suicidal thoughts and behavior among youths in the past 12 months.
- If adults or youths reported suicidal thoughts or behavior in the past 12 months, they were asked if these thoughts or behaviors were because of the COVID-19 pandemic.
- A series of questions related to the COVID-19 pandemic were added that asked about respondents’ perceptions of effects of the COVID-19 pandemic on their mental health, substance use, finances, living situation, and access to services.
This section discusses reasons for these questionnaire changes, anticipated issues for presenting estimates from these new items, and how these estimates were presented in 2020 NSDUH reports and tables. Section 6.2.3.2 discusses examples of how these questionnaire changes affected measures that were available in Quarter 1 and prior years.
A key issue for all measures that were available only in Quarter 4 was that estimates needed to be limited to Quarter 4 data. Therefore, estimates for these new measures that were limited to Quarter 4 data needed to be created using the Quarter 4 person-level analysis weights that were described in Sections 2.3.4.2 and 6.2.2.1.
6.2.3.1.1 Delivery of Virtual (Telehealth) Services
As discussed in Section 3.4.15 and in the 2020 key substance use and mental health indicators report (CBHSQ, 2021h), behavioral health care providers turned to virtual (telehealth) services as a means of delivering services while also limiting in-person contact during the COVID-19 pandemic. Changes to reimbursement policies during the COVID-19 pandemic also facilitated the delivery of virtual services. The addition of questions in Quarter 4 reflected this change to the delivery of services during the COVID-19 pandemic (see Section 2.2.2.3).
In the 2020 detailed tables, these Quarter 4 estimates for the receipt of virtual services were presented in the final tables within relevant sections. For example, tables showing estimates for the receipt of virtual substance use treatment services were presented within Section 5 of the detailed tables (CBHSQ, 2021e) for SUD and substance use treatment.
6.2.3.1.2 Suicidal Thoughts and Behavior (Including Because of the COVID-19 Pandemic)
Making suicide plans or attempting suicide may not necessarily be preceded by suicidal ideation (i.e., serious thoughts of suicide), especially among adolescents and young adults. For example, Rodway and colleagues (2020) found that death by suicide with minimal advance warning appeared to be relatively common among children, adolescents, and young adults aged 10 to 19 in the United Kingdom between 2014 and 2016. A study in the United States in 2001 to 2002 also found that the use of alcohol during periods of depression could be a marker for suicidal behavior among adolescents in the absence of reported suicidal ideation (Schilling et al., 2009). In addition, two studies of inpatients indicated that 10 minutes or less could elapse between the first current thought of suicide and an actual attempt among adult inpatients (Deisenhammer et al., 2009) and that impulsive reactivity to emotions was associated with the occurrence of suicide attempts among adolescent inpatients (Auerbach et al., 2017). A less recent study in Australia (Williams et al., 1980) also found that 40 percent of hospital patients in the study attempted suicide within 5 minutes or less of first thinking about it. A study with three large nonclinical samples (including military recruits, high school students, and college students) found that people who attempted suicide and those who considered suicide but did not attempt suicide exhibited a tendency to act impulsively in the face of negative emotions, but only those who attempted suicide exhibited a diminished ability to think through the consequences of their actions (Klonsky & May, 2010). Therefore, the changes in Quarter 4 to the skip logic for suicidal thoughts and behavior among adults and the added questions for suicidal thoughts and behavior among adolescents did not assume the co-occurrence of serious thoughts of suicide with suicide plans or attempts.
Trends in suicide attempts and deaths by suicide also have been increasing among adolescents (Miron et al., 2019; Wang et al., 2020). These trends in suicidal behaviors among adolescents are major public health concerns in the United States (Mojtabai et al., 2016; Mojtabai & Olfson, 2020). Vulnerable adolescent populations in particular may be at increased risk due to a complex combination of potentially negative family interactions, economic uncertainty, the stress and anxiety of living through a pandemic, and limited access to resources (Cohen & Bosk, 2020). Therefore, to better understand suicidal thoughts and behavior among adolescents, new questions were added to the NSDUH questionnaire for Quarter 4 of 2020.
This section focuses on the presentation of data for suicidal thoughts and behavior among adolescents and for suicidal thoughts and behavior among adults and adolescents because of COVID-19; these measures for 2020 were available only in Quarter 4. Data for suicidal thoughts and behavior among adults for any reason (i.e., not necessarily because of COVID-19) were available before Quarter 4, but the skip logic for suicide plans and attempts was changed for Quarter 4 (Sections 3.4.16.1 and 6.2.3.1).
Estimates for suicidal thoughts and behavior among adolescents and for suicidal thoughts and behavior among adults and adolescents because of COVID-19 were included in a new section of the 2020 detailed tables (Section 13). However, most estimates for suicidal thoughts and behavior among adolescents in 2020 because of COVID-19 were suppressed when denominators for percentages consisted of all adolescents who had specific suicidal thoughts or behaviors in the past 12 months (e.g., percentages for serious thoughts of suicide because of COVID-19 among adolescents who had serious thoughts of suicide for any reason).
6.2.3.1.3 Perceived Effects of the COVID-19 Pandemic
As noted in Section 6.1.2, negative effects of the COVID-19 pandemic on substance use and mental health have been cited in the news media and the research literature. However, questions from NSDUH before Quarter 4 did not ask respondents to attribute changes to the COVID-19 pandemic for their substance use; mental health; economic and housing situation; and access to medical care, substance use treatment, or mental health care. Therefore, new questions were added to the survey in Quarter 4 of 2020 about people’s perceptions of the effects of the COVID-19 pandemic on these different topic areas. Estimates for measures from these items were included in the new Section 13 of the 2020 detailed tables because data were available only from Quarter 4.
6.2.3.1.4 General Estimation Approach
As noted previously, person-level analysis weights specifically for Quarter 4 were created for producing estimates based on these data. These Quarter 4 estimates were weighted to represent the estimated numbers of people and percentages nationally based on data from the snapshot of 2020 during Quarter 4.
For example, the Quarter 4 data indicated that an estimated 2.2 million people aged 12 or older received virtual (telehealth) treatment in the past year for their substance use. This estimate for the past year was based on a question that asked respondents to recall their receipt of virtual substance use treatment services at any time from 12 months prior to their interview until their interview date. Respondents who were interviewed in November 2020, for example, were asked to recall whether they received virtual substance use treatment services during a 12-month period beginning in November 2019 until their interview date in November 2020. Nevertheless, national estimates for the receipt of virtual substance use treatment could have been different if these data had been available before Quarter 4 of 2020 (including more data from Quarters 2 and 3).
6.2.3.2 Presentation of Data Available from Quarters 1 and 4
Despite changes to the NSDUH questionnaire that were described in Sections 2.2.2.3, 2.2.2.4, and 6.2.3.1, the content of most NSDUH questions did not change between Quarters 1 and 4.143 Combining 2020 data from Quarters 1 and 4 was appropriate for analyses using questions that were asked in both quarters. Therefore, most 2020 estimates in NSDUH reports and tables were based on data from Quarters 1 and 4. No changes were needed to estimation procedures if the measures in 2020 were equivalent to those in prior years.
However, some questions were added or revised for Quarter 4 data collection. Some of these questions that were added to the 2020 NSDUH questionnaire in Quarter 4 were related to NSDUH measures from 2019 and prior years. For example, adults’ receipt of virtual mental health services in the past year was related to the concept from 2019 of adults’ receipt of any of the following mental health services in the past year: at inpatient locations, at outpatient locations, or taking prescription medication to treat a mental health condition. In these situations, the estimates for services other than virtual (telehealth) services were presented in the NSDUH tables and reports using all available data from Quarters 1 and 4. This method of estimating outcomes used all available information from the full sample and maintained definitions for 2020 that were comparable with those in prior years. For example, creating an aggregate measure of mental health service use among adults in the past year that used data from all quarters for the receipt of inpatient services, outpatient services, and prescription medication but did not include data from Quarter 4 for virtual mental health services maintained the comparability of this measure across years.
However, SAMHSA recognized that data users might want to compare estimates using new Quarter 4 questions (e.g., receipt of virtual [telehealth] services) with estimates for other related outcomes (e.g., other types of mental health services) that apply to the same reference period (e.g., the past 12 months). Data users may also want to see Quarter 1 in-person estimates for the existing outcomes. To facilitate these observations, SAMHSA decided to present selected estimates for Quarters 1 and 4 separately in the 2020 key substance use and mental health indicators report (CBHSQ, 2021h) for estimates related to new outcomes. As for the Quarter 4 weights mentioned previously, special person-level analysis weights were created for producing estimates based on Quarter 1 data (Sections 2.3.4.2 and 6.2.2.1). However, SAMHSA decided not to show these quarterly estimates in the 2020 detailed tables because the detailed tables are intended to be stand-alone sources of information. Consequently, users of the detailed tables could misinterpret results without the availability of associated text to explain the meaning of estimates.
Quarterly estimates shown in the 2020 key substance use and mental health indicators report (CBHSQ, 2021h) were limited to percentages. Estimated numbers of people with particular outcomes were not presented to reduce potential confusion. For example, weighted estimates for adults’ receipt of outpatient mental health services in the past year based on 2020 data from Quarter 1 would represent the national count or percentage of people who received outpatient mental health services in the past year; the estimate would be based on data from respondents who were interviewed in January through March 2020. Similarly, the corresponding weighted estimates for adults’ receipt of outpatient mental health services in the past year based on 2020 data from Quarter 4 also would represent the national count or percentage of people who received outpatient mental health services in the past year; unlike the Quarter 1 data, the Quarter 4 estimates would be based on data from respondents who were interviewed from October to December 2020. Although data from both quarters would provide national estimates, presenting two different estimates for numbers of people with a given outcome could be confusing to data users.
6.3 Special Analyses of Effects on the 2020 NSDUH Data
This section details initial analyses of the Quarter 4 data. Three additional factors that can affect outcomes also were considered for analyses, alone or in combination:
- seasonal effects,
- effects of COVID-19 or other societal events, and
- mode effects.
Therefore, this section details efforts to assess the effects of these additional factors.
Ultimately, the biggest effect on 2020 estimates is likely the absence of data from Quarter 2 and for most of Quarter 3, a period when states and localities had stay-at-home restrictions in place and people’s activities were especially affected during the COVID-19 pandemic. Unlike previous years, however, the 2020 NSDUH estimates capture information principally from the first and fourth quarters of 2020. Therefore, caution must be taken when interpreting estimates for 2020, such as for substance use in the past month, because of the very small amount of data that are available from April to September 2020.
6.3.1 Initial Analyses of the Quarter 4 Data
Quarter 4 data for the 2020 NSDUH were checked and analyzed because of the mix of web and in-person data after essentially a 6-month pause in data collection in response to the COVID-19 pandemic. This effort included the following checks:
- Were the new web processes working properly?
Extensive testing verified that all web processes and systems were working as planned. Therefore, this section does not discuss this issue.
- Were web and in-person respondents similar in demographic characteristics?
Reviews of unweighted frequencies confirmed that the demographic characteristics of the two groups were similar, except for education level. Web respondents tended to be more highly educated than in-person respondents (see Sections 2.3.4.2 and 6.2.2.2). This difference was not explained by the specific geographical areas where in-person interviews were deemed safe and permissible. As indicated previously, literature has noted a tendency for web respondents to have higher levels of education than the general population (Perrin & Atske, 2021). This finding led to a recommendation for adding education level to the poststratification adjustment of weights, as described in Section 6.2.2.2. This change was implemented in the production of person-level analysis weights.
- Did web and in-person paradata (e.g., response rates, item missingness) have similar characteristics?
The most notable difference between in-person and web-based data collection was the increased percentage of web respondents who started the interview but did not complete it, leaving any subsequent questions unanswered (i.e., break-offs). Regardless of data collection mode, respondents who broke off the interview before answering a sufficient number of substance use questions would fail the usability criteria (Section 2.3.1). Among all web respondents in Quarter 4 who started the interview, 96.8 percent met the usability criteria compared with 99.7 percent of corresponding in-person respondents in Quarter 4. Among the web respondents who provided usable data, 9.2 percent did not complete the interview.144 The corresponding percentage in Quarter 1 (in person only) was 0.05 percent (Section 2.3.4.2). Break-off rates in web surveys after questionnaire initiation can be in the double digits and are associated with questionnaire length (Vehovar & Cehovin, 2014); the average length for in-person interviews in NSDUH was 60 minutes.
When NSDUH respondents broke off the interview, data were missing (i.e., no response) for the remaining questions that respondents would have been eligible to be asked. The break-offs in the web data caused more item missingness in mental health data for adults than did responses of “don’t know” or “refused.”
A tabulation of break-offs by questionnaire section for various demographic subgroups and response patterns indicated that the break-offs were not explained by increased respondent burden among users of multiple substances. The likelihood of respondents to break off also was not related to arrests in the past 12 months; prior NSDUH data have shown arrests in the past 12 months to be associated with the occurrence of mental health issues among adults (Glasheen et al., 2012). Also, break-offs were less of an issue for youths than for adults.
Break-offs among adults were especially prevalent by the time that adults reached the sensitive mental health and adult depression sections. These sections covered mental health issues among adults related to psychological distress (and associated impairment in carrying out daily activities) and major depressive episode (MDE). The concern was that missing values in these sections contributed to bias, especially if the break-off occurred during the mental health or adult depression section. Therefore, a second set of break-off analysis weights was created that treated break-offs as nonresponse for the variables derived from the mental health, adult depression, and subsequent sections in the questionnaire that were not imputed. The break-off analysis weights are discussed more in Sections 2.3.4.2 and 6.2.2.3.
- Were Quarter 4 responses for outcomes of interest similar to those prior to Quarter 4, or did response values change in Quarter 4? Could changes be explained by COVID-19 or the addition of the web mode of data collection?
This section focuses on this class of evaluations to examine outcomes of interest. The evaluations aimed to inform the processing and documentation of the 2020 NSDUH data.
To investigate outcomes for Quarter 4 as soon as possible, preliminary analysis weights and imputations were used to approximate the production weights and imputation that would become available later. Using preliminary weights and imputations is a common practice for a “first look” at raw NSDUH data (i.e., not fully edited or imputed). Unusually large and counterintuitive differences between preliminary estimates in Quarter 4 and prior periods would suggest that the COVID-19 pandemic, changes in methodology, or both affected the Quarter 4 estimates.
As a starting point, detailed tables for a subset of substance use and mental health outcomes were produced that compared the following:
- Quarter 4 estimates in 2020 with those in Quarter 1 (Table 6.7),
- Quarter 4 estimates in 2020 and in Quarter 4 of prior years145 (Table 6.8), and
- combined 2020 data from Quarters 1 and 4 with corresponding combined data from Quarters 1 and 4 in prior years (Table 6.9).
Because of the preliminary nature of these data for 2020 and corresponding procedures used to create analogous estimates for other years, readers are reminded that data in Tables 6.7 to 6.9 are not official NSDUH estimates for any survey year. Rather, these data are presented for investigational purposes only.
Lifetime substance use estimates among people aged 12 or older have historically been stable or shown increases from new users (CBHSQ, 2020e). Two factors that can increase the numbers of lifetime substance users in the population over time are initiation of substance use and immigration of substance users into the United States (regardless of when they initiated use). Conversely, lifetime substance users will no longer be in the population if they die or emigrate out of the United States. Unless deaths or emigration among substance users outnumber new users and immigrant substance users, lifetime prevalence estimates would not be expected to decrease over time.
Therefore, significant differences in lifetime substance use estimates across periods of data collection—especially decreases—can indicate a break in comparability for these measures. Although the patterns varied somewhat by age group, unofficial estimates from Tables 6.7 to 6.9 showed marijuana and illicit drug use having a pattern of increases and cigarette use having a pattern of decreases. The preliminary prevalence of alcohol use appeared to be higher in Quarter 4 of 2020 than in prior periods, especially for past month use, but the prevalence of binge alcohol use in Quarter 4 was lower. The preliminary mental health outcomes showed little change over time. Increases in alcohol use in Quarter 4 of 2020 were consistent with other literature (Barbosa et al., 2020; Grossman et al., 2020; Pollard et al., 2020). However, the lack of apparent increases in the prevalence of mental health issues in Quarter 4 of 2020 was not consistent with a preliminary SAMHSA review of studies through November 30, 2020 (SAMHSA, 2021). This issue for mental health estimates contributed to further investigation of the break-off issue that was described previously.
6.3.2 Investigation of Seasonality Effects
In any survey year, seasonal variation in outcomes (i.e., seasonality) can cause estimates to vary by quarter. The goal of this investigation was to determine the likelihood that seasonality affected the Quarter 4 estimates for 2020.
To avoid the potential effects of the COVID-19 pandemic and changes in data collection modes in Quarters 1 and 4 of 2020, data were analyzed from 2016 to 2019, when all interviews were conducted in person and prior to the COVID-19 pandemic. For these comparisons of key substance use and mental health outcomes, quarterly estimates were produced using final analysis weights and imputations. The outcome prevalence estimates within a year were compared across all four quarters using a chi-square test for distributional differences. If the chi-square test was significant at the .05 level, then t tests were used to compare pairs of quarterly estimates. The results are summarized in Table 6.10.
As shown in Table 6.10, several differences among quarters in these years were significant, and some outcomes had more significant differences among quarters compared with other outcomes. For example, estimates for the following outcomes had 3 years with significant chi-square test results across quarters: past month marijuana use, past month illicit drug use, past month binge alcohol use, past year MDE among youths aged 12 to 17, and past year MDE with severe impairment among youths. However, significant quarterly differences were not consistent for these outcomes. In 2016, for example, estimates in Quarters 1 and 2 for past month marijuana use and past month illicit drug use were significantly different from estimates in Quarter 4. Estimates in Quarters 1 and 2 also were significantly different from the estimate in Quarter 3 for past month marijuana use but not for past month illicit drug use. In 2019, estimates for past month marijuana use and past month illicit drug use were significantly different between Quarters 1 and 2 (not significant for 2016). Estimates also were significantly different for these two outcomes in 2019 between Quarters 1 and 3. Estimates were significantly different between Quarters 3 and 4 in 2019 for past month marijuana use but not for past month illicit drug use. Estimates for past month binge alcohol use showed a different pattern of significant differences by quarter and year (e.g., significant differences in 2018 but not in 2017) compared with estimates for marijuana use and illicit drug use in the past month.
Estimates for past year alcohol use had significant chi-square test results for 2016 and 2018 but not for 2017 or 2019. A significant chi-square test result for a single year was not considered conclusive evidence of seasonal variation in estimates.
Taken together, there were few consistent patterns in how quarterly estimates differed across years. Consequently, seasonality per se was unlikely to be a major factor for explaining observed differences between 2020 estimates in Quarters 1 and 4 and between Quarter 4 estimates in 2020 and those in prior years.
6.3.3 Investigation of Effects of COVID-19 or Other Societal Events
Societal factors can influence human behavior and health. The societal factors and behavioral changes can be long term or short term. For example, the percentage of people aged 12 or older who used marijuana in the past year increased from 2002 to 2019. In contrast, the percentage of people aged 12 or older who smoked cigarettes in the past month decreased over this same period (CBHSQ, 2020a). The preliminary 2020 Quarter 4 estimates for these substances also reflected these general tendencies.
A factor that may have profoundly influenced human behavior and health in 2020 was the COVID-19 pandemic, but it was not the only societal factor. Other societal factors also could cause relatively sudden changes in the prevalence of substance use and mental health issues. In the context of survey research, sudden increases or decreases in the number of substance users or people with mental health issues would be expected to influence reported outcomes most proximal to the change, such as for the past month. Respondents’ reports of outcomes in the past year would be less sensitive to sudden decreases because most of the past year reference period would encompass a time before the decrease. However, sudden increases in numbers also could affect reports for the past year. Estimates for the lifetime period would be less sensitive to these sudden changes. For example, momentary decreases in numbers would not be expected to affect reports from people who already had an outcome in their lifetime. The alcohol use data in Tables 6.7 and 6.8 demonstrate this pattern, indicating that abrupt changes in prevalence did occur for some reason. The COVID-19 pandemic and other events in 2020 may have influenced estimates of outcomes, but the possibility cannot be dismissed that methodological issues contributed to observed differences between Quarter 4 of 2020 and Quarter 1 of 2020 or prior quarters.
To remove the confounding effects of changes to the mode of data collection and sampling for 2020, estimates from in-person interviews in Quarter 4 of 2020 were compared with the estimates from the same geographical areas in the 2019 NSDUH. Specifically, 84 segments used for in-person interviews in Quarter 4 were also used in the 2019 NSDUH because of the design feature that reuses 50 percent of the segments in the following year. The segments might not have been worked in 2019 Quarter 4, however. Samples in reused segments intended for Quarters 2 and 3 in 2020 were worked in Quarter 4 but would have been worked in Quarters 2 and 3 of 2019, respectively.
This analysis, summarized in Table 6.11, was conducted before 2020 Quarter 4 data collection was completed. Unweighted percentages were produced using in-person interviews from the common segments and compared across years. None of the percentages presented in Table 6.11 were significantly different between 2019 and 2020. This comparison was not repeated with final 2020 Quarter 4 data. The finding of no differences for the preliminary data that were checked suggests that COVID-19 may not have had a significant impact on these outcomes in these segments. However, because in-person data collection was permitted only in eligible segments based on COVID-19 infection rate metrics, these data do not provide conclusive evidence that aspects of the COVID-19 pandemic had no effect on outcomes for the general population.
6.3.4 Investigation of Survey Mode Effects
6.3.4.1 Nonmodel Investigations of Survey Mode Effects
Some in-person data collection took place in Quarter 4 of 2020, but decisions to collect in-person data were determined by COVID-19 infection metrics in sampled areas rather than through random assignment to in-person or web-based data collection. Nevertheless, the in-person data from Quarter 4 were available for comparison with web data. Using preliminary weights, prevalence estimates were reviewed from web and in-person interviews in Quarter 4 of 2020 for a common set of key substance use and mental health outcomes. These results are shown in Table 6.12 with three response values corresponding to yes, no, and missing. No statistical tests were performed comparing patterns by mode. Estimates were in the direction of being higher in the in-person data for the use of cigarettes, illicit drugs, and marijuana in the lifetime, past year, and past month periods or of being higher in the web data for alcohol use in the past year and past month. However, these patterns may not have been statistically significant and indicative of mode differences. Qualitatively, results for mental health outcomes shown in Table 6.12 did not appear to differ much by mode. As noted previously, however, missingness due to break-offs was an issue for the mental health data from web respondents.
Results of these direct comparisons were not conclusive because the mode of data collection was confounded with geography (i.e., the web and in-person interviews were conducted in separate segments based on local COVID-19 infection rates). Also, as noted in previous sections, the adult web respondents on average had a higher level of education compared with in-person respondents. Therefore, apparent differences in estimates by data collection mode could reflect differences in geography, local COVID-19 conditions, or the characteristics of people who chose to respond via the web. A model-based analysis was needed to address those potential differences.
6.3.4.2 Model-Based Investigations of Mode and the COVID-19 Pandemic Effects
As noted previously, effects of the COVID-19 pandemic on outcomes were confounded with potential effects of the different data collection modes in 2020. Nevertheless, two sets of analyses were conducted to tease out mode and COVID-19 effects through modeling, taking advantage of some limited availability of in-person data in Quarter 4 of 2020. Each set of analyses involved a series of logistic regressions with 21 key substance use and mental health outcomes shown in Table 6.13 as the dependent variables.
In the first set, each model regressed a key outcome of interest on mode and controlled for (1) the year of data collection; (2) COVID-19 positivity rate in the county where the segment was located; (3) several respondent-level characteristics, geographic-level characteristics, and household characteristics; 146 and (4) the log of preliminary weights to account for differences in selection probabilities and subsequent nonresponse adjustments. The dataset consisted of responses from respondents from all quarters of 2019 and from Quarter 4 of 2020. The resulting odds ratios showed which mode and which year had greater odds for the outcome variables.
There appeared to be effects of both mode and year on substance use outcomes, as shown in Table 6.13. The odds ratios for mode (with in-person interviews as the reference group) were statistically significant for lifetime and past year illicit drug use; lifetime and past month marijuana use; and lifetime, past year, and past month cigarette use. All significant odds ratios were less than 1, meaning that web-based data collection reduced the odds for substance use when the model controlled for the effects of other variables.
Table 6.13 also shows significant effects by year. However, the effects associated with year (Quarter 4 data for 2020 relative to full-year 2019 data) were in the opposite direction from mode effects. Specifically, data collection in Quarter 4 of 2020 was associated with higher odds of substance use (except for past month binge alcohol use) and any mental illness among adults.
Taken together, results from this first set of models suggest that the effects of mode and year (including onset of the COVID-19 pandemic in 2020) may negate each other to some extent. That is, the models indicate that the odds for substance use tended to be higher in Quarter 4 of 2020 than in 2019, but the web mode in Quarter 4 of 2020 tended to reduce the odds of substance use.
The second modeling effort was based on the risk ratios (relative risks) and compared (1) 2020 Quarter 1 in-person interviews (during the early stages of the COVID-19 pandemic, collected before statewide mandates went into effect147) with 2020 Quarter 4 in-person interviews (later in the COVID-19 pandemic) to assess effects of quarter as a proxy for COVID-19; (2) 2020 Quarter 4 in-person data with 2020 Quarter 4 web data to assess effects of mode; and (3) 2020 Quarter 1 (in person) with 2020 Quarter 4 (web) for the combined mode and quarter (COVID-19) effects. The dependent variables were 19 of the key outcome indicator variables used in the prior set of models described in Table 6.13. The COVID-19 variable for the risk ratio models was a binary indicator variable corresponding to Quarter 1 (reference group) versus Quarter 4. The mode variable was also a binary indicator variable, with web as the reference group. For the combined mode and quarter (COVID-19) models, the reference group was Quarter 4 web. The other predictors were categorical variables (raw versions) for the following: age, gender, race/ethnicity, education, employment status, and county type. The log-link models with or without preliminary weights (unadjusted for break-offs) were used to produce the risk ratios.
As shown in the first set of risk ratios in Table 6.14, in-person interviews in Quarter 4 (i.e., during the COVID-19 pandemic) had higher risk ratios for illicit drug use and marijuana use for all time frames (lifetime, past year, and past month) than in Quarter 1, when the COVID-19 pandemic was beginning to emerge in the United States. The results for MDE were reversed (i.e., the Quarter 4 data showed a 29 percent lower risk of MDE among adults than the Quarter 1 data). However, missing values due to break-offs may have contributed to that result for MDE. The effect of quarter increased for substance use as the reference period changed from lifetime to past year to past month use for illicit drugs and marijuana, with past month use showing the highest effects.
In-person interview data had higher risk ratios for illicit drug use, marijuana use, and cigarette use for all time frames (lifetime, past year, and past month) than for web data, as seen in the middle set of risk ratios in Table 6.14. Also, risk ratios were higher for lifetime and binge alcohol use among the in-person data than among the web data. The mode effect increased as the reporting period changed from lifetime to past year to past month use for the illicit drug, marijuana, and cigarette outcomes, with past month use showing the highest mode effects.
As noted in the previous set of odds ratio models and shown in the third set of risk ratios in Table 6.14, the mode and COVID-19 effects tended to work in the opposite directions. That is, the mode of data collection (i.e., web interviewing in Quarter 4 of 2020) could have depressed effects of the COVID-19 pandemic for these outcomes.
However, both sets of models were hampered by the limited number of in-person interviews in Quarter 4 of 2020. Therefore, caution is needed in drawing conclusions from these results. One aspect that cannot be determined is the exact nature of the mode effect on the Quarter 4 data. However, the vastly different response rates create the potential for the data collection mode to effect estimates (beyond the sociodemographic controls employed in weighting). As the COVID-19 pandemic subsides at some future point, differences between web and in-person responses can be measured in all areas, without the confounding effect of the COVID-19 pandemic. When the situation is more stable, the impact on survey estimates from the changing mix of web and in-person interview modes should also become more stable and less of a concern for measuring changes in behavior.
In summary, these modeling investigations were not extensive and were conducted using preliminary data and preliminary analysis weights. Therefore, results from these initial investigations may not generalize to all 2020 NSDUH data and to all population subgroups. Also, odds ratios and risk ratios from these investigations indicate the general direction of influences of the data collection mode and quarter for the outcomes that were investigated but cannot be used to construe what the 2020 estimates would have been if all data had been collected in person.
Data users should exercise caution when comparing the 2020 NSDUH estimates with estimates from prior years because the independent reasons for differences in estimates across years cannot be determined conclusively. The COVID-19 pandemic and the resulting addition of the web mode of data collection in Quarter 4 happened in tandem without the benefit of a controlled experiment to measure the effects of each. As mentioned previously, data collection in 2020 also differed from that in prior years because of the pause in data collection in the middle of 2020. For these reasons, SAMHSA decided not to compare 2020 estimates with those from prior years in the detailed tables and key substance use and mental health indicators report for the 2020 NSDUH (CBHSQ, 2021e, 2021h).
6.4 Summary
This chapter highlighted methodological issues experienced for the 2020 NSDUH that were related to changes in data collection methods necessitated by the COVID-19 pandemic. The chapter also noted reasons for these methodological changes and potential effects of the changes. Finally, the chapter discussed implications of these methodological changes for presentation of estimates for the 2020 NSDUH.
6.4.1 Background on the 2020 NSDUH and the COVID-19 Pandemic
Data collection methods for the 2020 NSDUH changed in several ways because of the COVID-19 pandemic:
- In-person data collection was suspended in all areas in mid-March 2020.
- No data were collected in Quarter 2, and only a small amount of data were collected in Quarter 3.
- In-person data collection resumed in limited areas in Quarter 4.
- A new web mode for data collection was offered in all other areas, resulting in 93 percent of Quarter 4 interviews being completed via the web.
- The mode of data collection for Quarter 4 was determined based on COVID-19 infection rate metrics instead of an experimental or other purposive mode assignment.
- New questions were added to the Quarter 4 questionnaire that were relevant to the COVID-19 pandemic, introducing analytic issues and the potential for context effects (see Section 3.4.6) for subsequent questions in the Quarter 4 interview.
These changes to 2020 NSDUH data collection affected imputation procedures, weighting procedures, presentation of the data, and analysis and interpretation of the data. Given these changes, interpretation of the 2020 NSDUH data must be made with caution.
As noted in Section 6.1.2, data from sources other than NSDUH that were collected in 2020 (primarily by web) suggested hypotheses that the COVID-19 pandemic negatively affected substance use and mental health outcomes in the United States. However, these web surveys used different samples, methods, and questions or scales in collecting data. Differences in substance use behavior and mental health estimates observed among these studies and differences between any of these surveys and NSDUH are subject to varying effects of survey errors. Also, COVID-19 infections and policy responses varied during 2020. Depending on when data were collected, findings might reflect a “snapshot” of substance use and mental health outcomes at a specific point in the COVID-19 pandemic.
6.4.2 Effects of Methodological Changes on Data Processing and Estimation
6.4.2.1 Effects on Imputation
Anticipated effects of changes to 2020 data collection that informed changes to the imputation procedures included differences in data collection modes, differences in item nonresponse, and quarterly differences due to the COVID-19 pandemic. Given the anticipated effects and available information, imputation procedures for 2020 were modified as follows:
- imputing Quarter 1 and 4 data separately to incorporate information about the quarter of data collection, and
- including interview mode as a covariate in the imputation models for Quarter 4 to take into account anticipated effects on data because of interview mode.
One observed outcome was that respondents in Quarter 4 (when interviews were predominately completed via the web) generally required imputation at a higher rate than Quarter 1 respondents (when interviews were exclusively conducted in person). A second observed outcome was variations in substance use patterns and the demographic and socioeconomic characteristics of respondents between Quarters 1 and 4. Imputing the data separately by quarter helped to ensure that these differences were reflected in the imputed data through the imputation model coefficients and selection of donors.
6.4.2.2 Effects on Person-Level Weighting
The following key modifications were made to the weighting procedures for the 2020 NSDUH:
- developing separate person-level analysis weights for Quarters 1 and 4,
- creating overall analysis weights for the combined data from Quarters 1 and 4 using a modified approach,
- including educational attainment in the last poststratification adjustment for Quarters 1 and 4, and
- developing additional person-level analysis weights to take into account missing data because of web break-offs.
Sections 6.2.2.1 through 6.2.2.3 provide an overview of the development of 2020 weights and reasons for each of these changes.
In general, these modifications to weighting procedures were intended to account for the disruption in data collection because of the COVID-19 pandemic (i.e., no data collection in Quarter 2 and extremely limited data collection in Quarter 3), to account for differences in data collection modes in Quarters 1 and 4 (i.e., exclusively in-person data collection in Quarter 1 and predominantly web-based data collection in Quarter 4). These modifications also accommodated analytic needs for presenting estimates from data that were available only from Quarter 4.
6.4.2.3 Effects on Presentation of Estimates
In addition to methodological changes, collecting 2020 NSDUH data during three distinct time periods had a major role in decisions on the presentation of 2020 estimates. These decisions included the following:
- not making statistical comparisons between 2020 estimates and those from prior years,
- using special formatting for the 2020 estimates in tables and figures that showed estimates for 2020 and prior years,
- including notes or other information in tables and reports to caution data users about making direct comparisons between estimates in 2020 and those from prior years, and
- limiting estimates for measures that were available only in Quarter 4 to Quarter 4 data using the Quarter 4 person-level analysis weights.
Questionnaire changes were made for Quarter 4 to cover topics on the use of virtual (telehealth) services, suicidal thoughts and behavior among youths; suicidal thoughts and behavior among youths and adults that were because of the COVID-19 pandemic; and respondents’ perceptions of effects of the COVID-19 pandemic on their mental health, substance use, finances, living situation, and access to services. Beginning in Quarter 4, all adult respondents also were asked if they made suicide plans or attempted suicide in the past 12 months, instead of these questions being restricted to adults who had serious thoughts of suicide in that period. Section 6.2.3.1 discussed reasons for these questionnaire changes, anticipated issues for presenting estimates from these new items, and how these estimates were presented in 2020 NSDUH reports and tables. Section 6.2.3.2 discussed how these questionnaire changes affected selected measures that were available in Quarter 1 and prior years.
6.4.3 Special Analyses of Effects of Mode Changes and the COVID-19 Pandemic
Initial analyses were conducted for the Quarter 4 data because of the methodological changes described previously. Changes in the 2020 data collection also prompted special analyses to assess the effects of seasonality, the COVID-19 pandemic or other societal factors, and the mode of data collection on key substance use and mental health estimates.
6.4.3.1 Initial Analyses of Quarter 4 Data
Quarter 4 data were checked because of the mix of web and in-person data after essentially a 6-month pause in data collection in response to the COVID-19 pandemic. This effort included the following checks:
- Were the new web processes working properly?
Testing verified that all systems were working as planned.
- Were web and in-person respondents similar in demographic characteristics?
Reviews of unweighted frequencies indicated similar demographic characteristics for the in-person and web respondents, with one exception. Consistent with the literature, web respondents completed higher levels of education than in-person respondents (see Sections 2.3.4.2 and 6.2.2.2). This difference was not explained by differences in educational attainment in the specific geographical areas where in-person interviews were feasible.
- Did web and in-person paradata, such as response rates and item missingness, have similar characteristics across modes?
The most notable difference between in-person and web-based data collection was the higher percentage of web respondents who started the interview but did not complete it, otherwise known as break-offs. Among all web respondents in Quarter 4 who started the interview, 96.8 percent met criteria for providing usable data compared with 99.7 percent of corresponding in-person respondents in Quarter 4. Among the web respondents who provided usable data, 9.2 percent did not complete the interview. Break-offs among adults were especially prevalent by the time that adults reached the sensitive mental health and adult depression sections, and some occurred during the mental health or adult depression sections.
- Were Quarter 4 responses for outcomes of interest similar to those prior to Quarter 4, or did response values change in Quarter 4? Could changes be explained by COVID-19 or the addition of the web mode of data collection?
Detailed tables for a subset of substance use and mental health outcomes based on preliminary 2020 data compared the following estimates:
- Quarter 4 estimates in 2020 with those in Quarter 1,
- Quarter 4 estimates in 2020 and in Quarter 4 of prior years, and
- combined 2020 data from Quarters 1 and 4 with corresponding combined data from Quarters 1 and 4 in prior years.
Although the patterns varied somewhat by age group, unofficial estimates from Tables 6.7 to 6.9 showed marijuana and illicit drug use having a pattern of increases and cigarette use having a pattern of decreases. The preliminary prevalence of alcohol use appeared to be higher in Quarter 4 of 2020 than in prior periods, especially for past month use, but the prevalence of binge alcohol use in Quarter 4 appeared to be lower. The preliminary mental health outcomes showed little change over time.
6.4.3.2 Investigation of Seasonality Effects
As shown in Table 6.10, several differences among quarters in these years were statistically significant, and some outcomes had more significant differences among quarters compared with other outcomes. Overall, however, there were few consistent patterns in how quarterly estimates differed across years, indicating that seasonality was unlikely to be a major factor for explaining observed differences between 2020 estimates in Quarters 1 and 4 and between Quarter 4 estimates in 2020 and prior years.
6.4.3.3 Investigation of Effects of COVID-19 or Other Societal Events
To assess the potential effect of the COVID-19 pandemic or other societal factors and to remove the confounding effects of changes to the mode of data collection and sampling for 2020, estimates from in-person interviews in Quarter 4 of 2020 were compared with the estimates from the same geographical areas in the 2019 NSDUH. This analysis, summarized in Table 6.11, was conducted before 2020 Quarter 4 data collection was completed. Unweighted percentages were produced using in-person interviews from the common segments and compared across years.
None of the percentages presented in Table 6.11 were significantly different between 2019 and 2020. This finding of no differences among the preliminary data suggests that COVID-19 may not have had a significant impact on these outcomes in these segments. However, because in-person data collection was permitted only in eligible segments based on COVID-19 infection rate metrics, these data do not provide conclusive evidence that aspects of the COVID-19 pandemic had no effect on outcomes for the general population.
6.4.3.4 Investigation of Survey Mode Effects
Two types of investigations of mode effects were conducted, a nonmodel-based approach and a model-based approach. For the former approach, prevalence estimates were reviewed from web and in-person interviews in Quarter 4 of 2020 for a common set of key substance use and mental health outcomes. As shown in Table 6.12, these results indicated estimates were in the direction of being higher in the in-person data for the use of cigarettes, illicit drugs, and marijuana in the lifetime, past year, and past month periods. Estimates were in the direction of being higher in the web data for alcohol use in the past year and past month. However, no statistical tests were performed comparing patterns by mode, so these directional results might not indicate true mode effects. These direct comparisons were also not conclusive because the mode of data collection was confounded with geography: web and in-person interviews were conducted in separate sets of segments.
For the latter approach, two sets of analyses were conducted to tease out mode and COVID-19 effects through modeling. In the first set, each model regressed a key outcome of interest on mode and controlled for (1) the year of data collection; (2) COVID-19 positivity rate in the county where the segment was located; (3) several respondent-level characteristics, geographic-level characteristics, and household characteristics; and (4) the log of preliminary weights to account for differences in selection probabilities and subsequent nonresponse adjustments. There appeared to be effects of both mode and year on substance use outcomes, as shown in Table 6.13. However, the effects associated with year (Quarter 4 data for 2020 relative to full-year 2019 data) were in the opposite direction from mode effects. Specifically, data collection in Quarter 4 of 2020 was associated with higher odds of substance use (except for past month binge alcohol use) and any mental illness among adults.
The second set was based on the risk ratios (relative risks) and compared (1) 2020 Quarter 1 in-person interviews during the early stages of the COVID-19 pandemic (before statewide mandates went into effect) with 2020 Quarter 4 in-person interviews (later during the COVID-19 pandemic) to assess effects of quarter as a proxy for COVID-19, (2) 2020 Quarter 4 in-person data with 2020 Quarter 4 web data to assess effects of mode, and (3) 2020 Quarter 1 (in person) with 2020 Quarter 4 (web) for the combined mode and quarter (COVID-19) effects. The dependent variables were 19 of the key outcome indicator variables used in the first set of models. As the results show in Table 6.14, in-person interviews in Quarter 4 (during the COVID-19 pandemic) had higher risk ratios for illicit drug use and marijuana use for all time frames—lifetime, past year, and past month—than in Quarter 1 (when COVID-19 was just emerging). Overall, in-person interview data had higher risk ratios for illicit drug use, marijuana use, and cigarette use for all time frames (lifetime, past year, and past month) than for web data. Caution should be exercised in drawing conclusions from these results because both sets of models were limited by the limited number of in-person interviews in Quarter 4 of 2020.
Data users also should exercise caution when comparing the 2020 NSDUH estimates with estimates from prior years because the independent reasons for differences in estimates across years cannot be determined conclusively. The COVID-19 pandemic and the resulting addition of the web mode of data collection in Quarter 4 happened in tandem without the benefit of a controlled experiment to measure the effects of each. As mentioned previously, data collection in 2020 also differed from that in prior years because of the pause in data collection in the middle of 2020. For these reasons, SAMHSA decided not to compare 2020 estimates with those from prior years in the detailed tables and key substance use and mental health indicators report for the 2020 NSDUH (CBHSQ, 2021e, 2021h).
Table 6.1 – Respondents Who Answered Don’t Know or Refused; by Data Collection Mode, Interview Type, and Number of Respondents Who Received Each Question, Unweighted Percentages, 2020
Question
Administration
(in person) |
Number of
Respondents
Who
Received
Question |
Web-Based Data Collection |
In-Person Data Collection |
Percentage of Respondents Who Answered Don’t Know
or Refused |
Percentage of Respondents Who Answered Don’t Know
or Refused |
| Mean |
Min |
Q1 |
Median |
Q3 |
Max |
Mean |
Min |
Q1 |
Median |
Q3 |
Max |
| Self-Administered |
≥ 1 |
1.95 |
0.00 |
0.00 |
0.03 |
0.64 |
100.00 |
3.07 |
0.00 |
0.00 |
0.08 |
1.33 |
100.00 |
| ≥ 25 |
0.86 |
0.00 |
0.00 |
0.20 |
0.83 |
26.67 |
1.84 |
0.00 |
0.00 |
0.51 |
1.73 |
85.71 |
| ≥ 50 |
0.75 |
0.00 |
0.00 |
0.24 |
0.80 |
16.48 |
1.64 |
0.00 |
0.15 |
0.61 |
1.72 |
62.00 |
| ≥ 100 |
0.69 |
0.00 |
0.04 |
0.26 |
0.74 |
16.48 |
1.53 |
0.00 |
0.19 |
0.60 |
1.66 |
34.58 |
Interviewer
Administered |
≥ 1 |
2.95 |
0.00 |
0.00 |
0.64 |
3.48 |
33.33 |
1.07 |
0.00 |
0.00 |
0.01 |
0.27 |
36.62 |
| ≥ 25 |
2.36 |
0.00 |
0.09 |
1.06 |
3.54 |
13.25 |
1.22 |
0.00 |
0.00 |
0.04 |
0.32 |
36.62 |
| ≥ 50 |
2.34 |
0.00 |
0.19 |
1.08 |
3.54 |
13.25 |
1.31 |
0.00 |
0.00 |
0.05 |
0.35 |
36.62 |
| ≥ 100 |
2.17 |
0.00 |
0.21 |
1.07 |
3.48 |
11.03 |
1.36 |
0.00 |
0.00 |
0.06 |
0.35 |
36.62 |
Max = maximum; Min = minimum; Q = quartile.
NOTE: Unweighted percentages are calculated separately for each variable with a given set of characteristics (e.g., self-administered questions received by at least one respondent). The statistics (e.g., mean, median) associated with the distribution across all variables with the same set of characteristics is then calculated.
Source: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2020. |
|---|
Table 6.2 – Impact of Break-Offs on Imputation Rates; Quarter 4, 2020
Questionnaire
Section |
Question
Administration
(in person) |
Number of
Variables
Imputed |
Imputation Rate: All Quarter 4 Respondents |
Imputation Rate: Quarter 4 Respondents
Excluding Break-Offs |
| Mean |
Min |
Max |
Mean |
Min |
Max |
| DSM-5 SUD1 |
Self-Administered |
22 |
12.11% |
1.14% |
42.45% |
6.43% |
0.10% |
41.50% |
| Emerging Issues2 |
Self-Administered |
10 |
8.12% |
6.93% |
12.18% |
0.23% |
0.00% |
0.41% |
| Household Roster |
Interviewer Administered |
6 |
8.66% |
7.61% |
9.60% |
0.69% |
0.35% |
1.09% |
| Income |
Interviewer Administered |
10 |
14.26% |
11.49% |
21.32% |
3.94% |
1.13% |
9.27% |
| Health Insurance |
Interviewer Administered |
6 |
11.69% |
10.89% |
13.88% |
1.67% |
0.81% |
4.00% |
DSM-5 = Diagnostic and Statistical Manual of Mental Disorders, 5th edition; Max = maximum; Min = minimum; SUD = substance use disorder.
NOTE: Imputation rates refer to weighted percentages corresponding to the percentage of respondents in an imputation-revised domain that required imputation (e.g., the percentage of final imputed past month alcohol users whose frequency of use in the past 30 days required imputation). Statistics shown in the table are for the weighted rates across all variables in a given section.
1 Most SUD questions in Quarter 4 were in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) SUD section. The emerging issues section contained additional DSM-5 SUD questions for craving (all substances), marijuana withdrawal, and tranquilizer withdrawal.
2 Individual variables corresponding to DSM-5 SUD questions in the emerging issues section were not imputed.
Source: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2020. |
Table 6.3 – Weighted Raw Substance Use Prevalence; Relative Percent Change, between Quarter 1 and Quarter 4, 2020
| Substance |
Relative Percent Change: (Quarter 4 – Quarter 1)/Quarter 1 |
| Past Month Use |
Past Year Use |
| TOBACCO PRODUCTS |
−16.7% |
−15.7% |
| ALCOHOL |
−4.2% |
−4.2% |
| ILLICIT DRUGS |
5.7% |
1.7% |
| Marijuana |
7.2% |
2.8% |
| Cocaine |
−9.1% |
−2.2% |
| Heroin |
−14.4% |
12.3% |
| Hallucinogens |
−8.6% |
19.4% |
| Inhalants |
−1.0% |
−0.5% |
| Methamphetamine |
81.7% |
40.6% |
| Misuse of Psychotherapeutics |
1.2% |
−9.0% |
| Pain Relievers |
−15.5% |
−3.5% |
| Stimulants |
−22.5% |
−29.5% |
| Tranquilizers |
46.6% |
−0.3% |
| Sedatives |
75.7% |
63.4% |
| Source: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2020. |
Table 6.4 – Weighted Raw Demographic Characteristics; Relative Percent Change, between Quarter 1 and Quarter 4, 2020
| Characteristic |
Relative Percent Change: (Quarter 4 – Quarter 1)/Quarter 1 |
| Hispanic or Latino |
1.0% |
| Employed Full Time or Part Time1 |
−9.2% |
| Born in the United States |
2.1% |
| College Graduate2 |
0.4% |
| Married1 |
−3.2% |
| Family Income ≥ $50,000 |
−4.2% |
| Receiving Any Government Assistance |
5.4% |
| Receiving Any Health Insurance |
−1.2% |
1 Among people aged 15 or older.
2 Among people aged 18 or older.
Source: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2020. |
Table 6.5 – Unweighted Distributions for Educational Attainment: Among Adults; Percentages, 2016-2020
Educational
Attainment |
2016 |
2017 |
2018 |
2019 |
Quarter 1,
2020 |
Quarter 4,
2020 |
| Less Than High School |
12.7 |
12.2 |
12.2 |
11.7 |
12.4 |
6.3 |
| High School Graduate |
26.1 |
25.9 |
26.1 |
26.2 |
24.7 |
18.6 |
| Some College or Associate Degree |
34.4 |
33.8 |
33.8 |
33.6 |
33.4 |
30.1 |
| College Graduate |
26.8 |
28.1 |
27.9 |
28.5 |
29.5 |
45.0 |
| Source: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2016-2020. |
Table 6.6 – NSDUH and ACS Weighted Distributions of Educational Attainment: Among Adults; Percentages, 2016-2020
Educational
Attainment |
2016 |
2017 |
2018 |
2019 |
2020 |
| NSDUH1 |
ACS |
NSDUH1 |
ACS |
NSDUH1 |
ACS |
NSDUH1 |
ACS |
Quarter 1
NSDUH1 |
Quarter 4
NSDUH1 |
| Less Than High School |
13.2 |
12.6 |
12.5 |
12.1 |
12.4 |
11.8 |
12.0 |
11.5 |
11.9 |
8.4 |
| High School Graduate |
25.0 |
27.6 |
24.2 |
27.7 |
24.7 |
27.5 |
24.3 |
27.6 |
23.4 |
19.5 |
| Some College or Associate Degree |
31.0 |
31.0 |
31.1 |
30.8 |
31.0 |
30.7 |
30.8 |
30.3 |
30.7 |
28.8 |
| College Graduate |
30.8 |
28.7 |
32.2 |
29.4 |
31.9 |
30.1 |
32.9 |
30.6 |
34.0 |
43.3 |
ACS = American Community Survey; NSDUH = National Survey on Drug Use and Health.
1 Weighted distributions for 2016-2019 NSDUHs are based on final analysis weights. Weighted distributions for Quarter 1, 2020, and Quarter 4, 2020, are based on nonresponse adjusted weights (no poststratification adjustment is included).
Sources: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2016-2020; American Community Survey, 2016-2019. |
Table 6.7 – Differences in Quarterly Percentages; Quarter 4 – Quarter 1, 2017-2020, Aged 12 or Older
| Outcome |
Weighted Data1 |
Quarter 4 –
Quarter 1,
2017 |
Quarter 4 –
Quarter 1,
2018 |
Quarter 4 –
Quarter 1,
2019 |
Quarter 4 –
Quarter 1,
2020 |
| Lifetime Marijuana Use |
0.7 |
0.4 |
3.2 |
2.1 |
| Past Year Marijuana Use |
1.5 |
1.1 |
0.7 |
0.6 |
| Past Month Marijuana Use |
0.9 |
1.1 |
0.9 |
0.8 |
| Lifetime Cigarette Use |
−0.9 |
−0.4 |
0.3 |
−3.9 |
| Past Year Cigarette Use |
−0.3 |
−0.7 |
1.3 |
−5.1 |
| Past Month Cigarette Use |
−0.1 |
−0.2 |
1.4 |
−4.7 |
| Lifetime Alcohol Use |
0.4 |
−0.3 |
0.5 |
1.4 |
| Past Year Alcohol Use |
−0.1 |
−1.9 |
0.1 |
2.5 |
| Past Month Alcohol Use |
0.0 |
−1.4 |
−0.2 |
4.1 |
| Lifetime Illicit Drug Use |
0.2 |
0.9 |
3.3 |
1.4 |
| Past Year Illicit Drug Use |
1.0 |
1.2 |
0.8 |
0.8 |
| Past Month Illicit Drug Use |
0.8 |
1.3 |
0.9 |
0.9 |
| Past Month Binge Alcohol Use |
−0.5 |
−0.1 |
0.1 |
−2.6 |
| Past Year Any Mental Illness (18 or Older) |
0.9 |
1.5 |
0.8 |
0.3 |
| Past Year Serious Mental Illness (18 or Older) |
0.4 |
0.1 |
0.9 |
−0.3 |
| Past Year Major Depressive Episode (18 or Older) |
0.0 |
0.8 |
0.7 |
−0.1 |
| Past Year Major Depressive Episode with Severe Impairment (18 or Older) |
0.1 |
0.4 |
0.5 |
−0.3 |
| Serious Thoughts of Suicide in Past Year (18 or Older) |
0.4 |
0.3 |
0.1 |
−0.2 |
| Made Any Suicide Plans in Past Year (18 or Older) |
0.1 |
0.2 |
0.0 |
−0.2 |
| Attempted Suicide in Past Year (18 or Older) |
−0.1 |
0.0 |
0.0 |
−0.2 |
1 Standard preliminary weights (inverse-probability weights separately adjusted for nonresponse in each collection quarter at dwelling unit and person levels using reweighting classes and then divided by 2) were used for all years for consistency.
Source: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2017-2020. |
Table 6.8 – Quarter 4 Percentages, 2016-2020, Aged 12 or Older
| Outcome |
Weighted Data1 |
| 2016 |
2017 |
2018 |
2019 |
2020 |
| Lifetime Marijuana Use |
45.7 |
47.1 |
47.4 |
| Past Year Marijuana Use |
16.1 |
16.6 |
17.2 |
| Past Month Marijuana Use |
11.0 |
11.5 |
| Lifetime Cigarette Use |
50.5 |
| Past Year Cigarette Use |
13.6 |
| Past Month Cigarette Use |
10.7 |
| Lifetime Alcohol Use |
80.6 |
80.6 |
80.6 |
81.8 |
| Past Year Alcohol Use |
67.5 |
| Past Month Alcohol Use |
54.8 |
| Lifetime Illicit Drug Use |
50.0 |
51.3 |
51.4 |
| Past Year Illicit Drug Use |
19.8 |
20.0 |
20.9 |
| Past Month Illicit Drug Use |
12.1 |
12.4 |
13.1 |
| Past Month Binge Alcohol Use |
19.5 |
| Past Year Any Mental Illness (18 or Older) |
19.5 |
19.7 |
20.7 |
20.6 |
| Past Year Serious Mental Illness (18 or Older) |
4.9 |
4.7 |
5.6 |
5.2 |
| Past Year Major Depressive Episode (18 or Older) |
7.5 |
8.0 |
8.3 |
| Past Year Major Depressive Episode with Severe Impairment (18 or Older) |
4.9 |
5.4 |
5.6 |
| Serious Thoughts of Suicide in Past Year (18 or Older) |
4.7 |
4.5 |
4.6 |
4.7 |
| Made Any Suicide Plans in Past Year (18 or Older) |
1.2 |
1.4 |
1.4 |
1.4 |
1.1 |
| Attempted Suicide in Past Year (18 or Older) |
0.3 |
1 Standard preliminary weights (inverse-probability weights separately adjusted for nonresponse in each collection quarter at dwelling unit and person levels using reweighting classes and then divided by 2) were used for all years for consistency.
a Significantly different from 2020 estimate at the .05 level.
Source: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2016-2020. |
Table 6.9 – Percentages for Quarter 1 and Quarter 4 Combined, 2018-2020, Aged 12 or Older
| Outcome |
Weighted Data1 |
| 2018 |
2019 |
2020 |
| Lifetime Marijuana Use |
45.5 |
46.4 |
| Past Year Marijuana Use |
16.3 |
16.9 |
| Past Month Marijuana Use |
10.5 |
11.1 |
| Lifetime Cigarette Use |
52.5 |
| Past Year Cigarette Use |
16.2 |
| Past Month Cigarette Use |
13.1 |
| Lifetime Alcohol Use |
80.8 |
80.3 |
81.1 |
| Past Year Alcohol Use |
66.3 |
| Past Month Alcohol Use |
52.7 |
| Lifetime Illicit Drug Use |
49.6 |
50.7 |
| Past Year Illicit Drug Use |
19.6 |
20.5 |
| Past Month Illicit Drug Use |
12.0 |
12.6 |
| Past Month Binge Alcohol Use |
20.8 |
| Past Year Any Mental Illness (18 or Older) |
20.3 |
20.4 |
| Past Year Serious Mental Illness (18 or Older) |
5.1 |
5.4 |
| Past Year Major Depressive Episode (18 or Older) |
8.4 |
| Past Year Major Depressive Episode with Severe Impairment (18 or Older) |
5.7 |
| Serious Thoughts of Suicide in Past Year (18 or Older) |
4.6 |
4.8 |
| Made Any Suicide Plans in Past Year (18 or Older) |
1.3 |
1.4 |
1.2 |
| Attempted Suicide in Past Year (18 or Older) |
0.4 |
1 Standard preliminary weights (inverse-probability weights separately adjusted for nonresponse in each collection quarter at dwelling unit and person levels using reweighting classes and then divided by 2) were used for all years for consistency.
a Significantly different from 2020 estimate at the .05 level.
Source: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2018-2020. |
Table 6.10 – Summary of Quarterly Differences in Outcomes, 2016-2019
| Outcome |
Number of
Years with
Significant
Chi-Square Tests |
2016
Significant
Differences
between
Quarters |
2017
Significant
Differences
between
Quarters |
2018
Significant
Differences
between
Quarters |
2019
Significant
Differences
between
Quarters |
| Past Month Marijuana Use |
3 |
Q1 and Q2 vs. Q3 and Q4 |
Q1 vs. Q2 and Q4 |
|
Q3 vs. others and Q1 vs. Q2 |
| Past Month Illicit Drug Use |
3 |
Q1 and Q2 vs. Q4 |
Q1 vs. Q2 and Q4 |
|
Q1 vs. Q2 and Q3 |
| Past Month Binge Alcohol Use |
3 |
Q1 and Q3 vs. Q2 and Q4 |
|
Q3 vs. Q2 and Q4 |
Q1 vs. Q2, Q2 vs. Q3, Q3 vs. Q4 |
| Past Year Major Depressive Episode (12-17) |
3 |
Q1 vs. Q2, Q2 vs. Q3, Q3 vs. Q4 |
|
Q4 vs. others |
Q3 vs. others |
| Past Year Major Depressive Episode with Severe Impairment (12-17) |
3 |
Q1 and Q3 vs. Q2 |
|
Q1 and Q3 vs. Q4 |
Q3 vs. others |
| Past Month Alcohol Use |
2 |
Q1 and Q3 vs. Q4 |
|
Q2 and Q4 vs. Q3 |
|
| Lifetime Marijuana Use |
1 |
|
|
|
Q4 vs. others |
| Past Year Marijuana Use |
1 |
|
Q1 vs. Q4 |
|
|
| Lifetime Cigarette Use |
1 |
|
|
|
Q2 and Q3 vs. Q4 |
| Past Year Cigarette Use |
1 |
Q2 vs. others |
|
|
|
| Past Month Cigarette Use |
1 |
Q2 vs. others |
|
|
|
| Past Year Alcohol Use |
1 |
|
|
Q1 vs. Q2 and Q4 |
|
| Lifetime Illicit Drug Use |
1 |
|
|
|
Q1 and Q3 vs. Q4 |
| Lifetime Alcohol Use |
0 |
|
|
|
|
| Past Year Illicit Drug Use |
0 |
|
|
|
|
| Past Year Any Mental Illness (18 or Older) |
0 |
|
|
|
|
| Past Year Serious Mental Illness (18 or Older) |
0 |
|
|
|
|
| Past Year Major Depressive Episode (18 or Older) |
0 |
|
|
|
|
| Past Year Major Depressive Episode with Severe Impairment (18 or Older) |
0 |
|
|
|
|
| Serious Thoughts of Suicide in Past Year (18 or Older) |
0 |
|
|
|
|
| Made Any Suicide Plans in Past Year (18 or Older) |
0 |
|
|
|
|
| Attempted Suicide in Past Year (18 or Older) |
0 |
|
|
|
|
Q1 = Quarter 1; Q2 = Quarter 2; Q3 = Quarter 3; Q4 = Quarter 4.
Source: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2016-2019. |
Table 6.11 – Unweighted Comparison for Selected Substance Use and Mental Health Outcomes; Quarter 2 to Quarter 4, 2019, and In-Person Quarter 4, 2020
| Outcome |
Quarter 2 to Quarter
4, 2019 |
Quarter 4, 2020
(in person) |
Test of Differences |
| % |
SE % |
% |
SE % |
T-statistic |
p-value |
| Lifetime Marijuana Use |
47.2 |
1.78 |
50.8 |
2.63 |
−1.42 |
0.1584 |
| Past Year Marijuana Use |
23.9 |
1.59 |
24.7 |
2.26 |
−0.33 |
0.7411 |
| Past Month Marijuana Use |
16.4 |
1.38 |
16.5 |
1.66 |
−0.08 |
0.9331 |
| Lifetime Cigarette Use |
47.7 |
2.19 |
46.1 |
2.26 |
0.68 |
0.4987 |
| Past Year Cigarette Use |
20.7 |
1.81 |
18.3 |
1.51 |
1.23 |
0.2226 |
| Past Month Cigarette Use |
15.6 |
1.76 |
14.2 |
1.53 |
0.70 |
0.4884 |
| Lifetime Alcohol Use |
72.7 |
1.61 |
69.8 |
2.11 |
1.23 |
0.2221 |
| Past Year Alcohol Use |
60.0 |
2.03 |
57.7 |
2.05 |
0.97 |
0.3335 |
| Past Month Alcohol Use |
45.1 |
1.83 |
44.5 |
2.27 |
0.29 |
0.7722 |
| Lifetime Illicit Drug Use |
52.6 |
1.81 |
55.3 |
2.50 |
−1.08 |
0.2813 |
| Past Year Illicit Drug Use |
27.8 |
1.62 |
28.0 |
2.45 |
−0.07 |
0.9404 |
| Past Month Illicit Drug Use |
17.9 |
1.35 |
17.8 |
1.81 |
0.04 |
0.9711 |
| Past Month Binge Alcohol Use |
20.1 |
1.33 |
19.2 |
1.58 |
0.50 |
0.6187 |
| Past Year Any Mental Illness (18 or Older) |
27.3 |
1.69 |
29.7 |
2.19 |
−0.96 |
0.3416 |
| Past Year Serious Mental Illness (18 or Older) |
8.0 |
1.02 |
7.8 |
1.28 |
0.12 |
0.9049 |
| Past Year Major Depressive Episode (18 or Older) |
11.9 |
1.09 |
11.7 |
1.60 |
0.06 |
0.9522 |
| Past Year Major Depressive Episode with Severe Impairment (18 or Older) |
7.5 |
0.95 |
7.9 |
1.35 |
−0.20 |
0.8414 |
| Past Year Suicidal Thoughts (18 or Older) |
7.6 |
1.13 |
7.0 |
1.25 |
0.45 |
0.6501 |
| Past Year Suicide Plans (18 or Older) |
1.8 |
0.50 |
1.9 |
0.61 |
−0.14 |
0.8913 |
| Past Year Suicide Attempts (18 or Older) |
0.8 |
0.38 |
0.5 |
0.30 |
0.63 |
0.5275 |
SE = standard error.
Source: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2019-2020. |
Table 6.12 – Preliminary Weighted Distribution of Responses for Outcomes by Mode, Quarter 4, 2020
| Outcome1 |
In-Person Data Collection |
Web-Based Data Collection |
| Yes |
No |
Missing |
Yes |
No |
Missing |
| Lifetime Marijuana Use |
54.0 |
46.0 |
N/A |
47.1 |
52.9 |
0.0 |
| Past Year Marijuana Use |
21.1 |
78.7 |
0.1 |
17.0 |
82.9 |
0.1 |
| Past Month Marijuana Use |
15.8 |
84.1 |
0.1 |
11.2 |
88.7 |
0.1 |
| Lifetime Cigarette Use |
55.9 |
44.1 |
N/A |
50.1 |
49.9 |
N/A |
| Past Year Cigarette Use |
18.4 |
81.3 |
0.3 |
13.3 |
86.7 |
0.0 |
| Past Month Cigarette Use |
15.3 |
84.7 |
N/A |
10.5 |
89.5 |
0.0 |
| Lifetime Alcohol Use |
82.3 |
17.7 |
N/A |
81.8 |
18.2 |
0.0 |
| Past Year Alcohol Use |
61.9 |
37.9 |
0.2 |
67.8 |
32.1 |
0.0 |
| Past Month Alcohol Use |
48.2 |
51.6 |
0.2 |
55.1 |
44.8 |
0.0 |
| Lifetime Illicit Drug Use |
58.1 |
40.9 |
1.0 |
51.1 |
47.8 |
1.2 |
| Past Year Illicit Drug Use |
24.3 |
74.4 |
1.3 |
20.7 |
78.7 |
0.7 |
| Past Month Illicit Drug Use |
17.0 |
81.5 |
1.5 |
12.8 |
86.4 |
0.8 |
| Past Month Binge Alcohol Use |
21.0 |
77.5 |
1.4 |
19.4 |
80.2 |
0.4 |
| Past Year Any Mental Illness (18 or Older) |
21.8 |
76.7 |
1.5 |
20.6 |
72.7 |
6.7 |
| Past Year Serious Mental Illness (18 or Older) |
4.9 |
93.2 |
1.9 |
5.3 |
87.3 |
7.4 |
| Past Year Major Depressive Episode (18 or Older) |
6.2 |
92.7 |
1.1 |
7.9 |
85.8 |
6.3 |
| Past Year Major Depressive Episode with Severe Impairment (18 or Older) |
3.5 |
95.4 |
1.1 |
5.4 |
88.3 |
6.3 |
| Past Year Suicidal Thoughts (18 or Older) |
4.3 |
95.3 |
0.4 |
4.5 |
90.0 |
5.5 |
| Past Year Suicide Plans (18 or Older) |
1.1 |
98.5 |
0.4 |
1.1 |
93.5 |
5.4 |
| Past Year Suicide Attempts (18 or Older) |
0.5 |
99.1 |
0.4 |
0.3 |
94.3 |
5.4 |
N/A = not applicable because no respondents had missing data. Estimates of 0.0 percent indicate that some respondents had missing data, but the percentage rounded to zero when shown to one decimal place.
1 Outcomes were estimated using standard preliminary weights (inverse-probability weights separately adjusted for nonresponse in each collection quarter at dwelling unit and person levels using reweighting classes and then divided by 2).
Source: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2020. |
Table 6.13 – Odds Ratios for Mode and for Year and 95% Confidence Intervals from Logistic Regression Models of 21 Outcomes
| Outcome |
Mode |
Year |
Odds
Ratio |
Lower
Limit |
Upper
Limit |
Odds
Ratio |
Lower
Limit |
Upper
Limit |
| Lifetime Illicit Drug Use |
0.69 |
0.92 |
1.28 |
1.67 |
| Past Year Illicit Drug Use |
0.71 |
0.98 |
1.22 |
1.67 |
| Past Month Illicit Drug Use |
0.84 |
0.69 |
1.01 |
1.23 |
1.76 |
| Lifetime Marijuana Use |
0.71 |
0.95 |
1.31 |
1.72 |
| Past Year Marijuana Use |
0.85 |
0.72 |
1.01 |
1.27 |
1.73 |
| Past Month Marijuana Use |
0.67 |
0.996 |
1.24 |
1.79 |
| Lifetime Alcohol Use |
0.88 |
0.72 |
1.08 |
1.19 |
0.97 |
1.46 |
| Past Year Alcohol Use |
1.03 |
0.88 |
1.19 |
1.07 |
0.92 |
1.25 |
| Past Month Alcohol Use |
0.97 |
0.85 |
1.12 |
1.05 |
0.92 |
1.21 |
| Past Month Binge Alcohol Use |
0.94 |
0.81 |
1.10 |
0.74 |
0.98 |
| Lifetime Cigarette Use |
0.69 |
0.90 |
1.08 |
0.95 |
1.22 |
| Past Year Cigarette Use |
0.71 |
0.998 |
0.93 |
0.80 |
1.09 |
| Past Month Cigarette Use |
0.63 |
0.92 |
0.91 |
0.76 |
1.10 |
| Past Year Serious Mental Illness (18 or Older) |
0.91 |
0.73 |
1.14 |
1.15 |
0.94 |
1.42 |
| Past Year Any Mental Illness (18 or Older) |
0.89 |
0.76 |
1.05 |
1.08 |
1.46 |
| Past Year Major Depressive Episode (18 or Older) |
1.02 |
0.80 |
1.30 |
1.06 |
0.84 |
1.33 |
| Past Year Major Depressive Episode with Severe Impairment (18 or Older) |
1.08 |
0.82 |
1.43 |
1.06 |
0.82 |
1.36 |
| Past Year Suicidal Thoughts (18 or Older) |
0.97 |
0.76 |
1.25 |
1.10 |
0.88 |
1.38 |
| Past Year Suicide Plans (18 or Older) |
1.33 |
0.77 |
2.30 |
0.87 |
0.51 |
1.50 |
| Past Year Suicide Attempts (18 or Older) |
1.41 |
0.61 |
3.26 |
0.52 |
0.23 |
1.18 |
| Past Year Major Depressive Episode (12 to 17) |
0.73 |
0.51 |
1.03 |
1.30 |
0.95 |
1.76 |
NOTE: The upper limit of the 95 percent confidence interval for an odds ratio is shown to three decimal places if the odds ratio is significantly different from 1.0 but the upper limit of the confidence interval would round to 1.00 if shown to two decimal places.
a Significantly different from 1.0 at the .05 level.
Source: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2020. |
Table 6.14 – Weighted Log-Link Regression Model Risk Ratios for Effects of COVID-19 and Mode of Data Collection on Quarterly Estimates of 19 Outcomes in 2020
| Outcome |
Quarter 4 In-Person vs.
Quarter 1 In-Person
Data Collection |
Quarter 4 In-Person vs.
Quarter 4 Web-Based
Data Collection |
Quarter 1 In-Person vs.
Quarter 4 Web-Based
Data Collection |
| Risk Ratio |
p-value |
Risk Ratio |
p-value |
Risk Ratio |
p-value |
| Lifetime Illicit Drug Use |
0.00 |
0.00 |
1.01 |
0.65 |
| Past Year Illicit Drug Use |
0.02 |
0.02 |
0.98 |
0.61 |
| Past Month Illicit Drug Use |
0.00 |
0.00 |
0.94 |
0.17 |
| Lifetime Marijuana Use |
0.00 |
0.00 |
1.00 |
0.81 |
| Past Year Marijuana Use |
0.00 |
0.00 |
0.99 |
0.80 |
| Past Month Marijuana Use |
0.00 |
0.00 |
0.94 |
0.17 |
| Lifetime Alcohol Use |
1.02 |
0.27 |
0.01 |
0.02 |
| Past Year Alcohol Use |
0.96 |
0.20 |
0.98 |
0.50 |
1.02 |
0.16 |
| Past Month Alcohol Use |
0.97 |
0.49 |
0.97 |
0.52 |
1.00 |
0.96 |
| Past Month Binge Alcohol Use |
1.02 |
0.82 |
0.04 |
0.00 |
| Lifetime Cigarette Use |
1.02 |
0.54 |
0.00 |
0.00 |
| Past Year Cigarette Use |
1.03 |
0.74 |
0.01 |
0.00 |
| Past Month Cigarette Use |
1.03 |
0.80 |
0.02 |
0.00 |
| Past Year Any Mental Illness (18 or Older) |
1.01 |
0.92 |
1.03 |
0.81 |
0.99 |
0.81 |
| Past Year Serious Mental Illness (18 or Older) |
0.82 |
0.23 |
0.86 |
0.34 |
1.05 |
0.46 |
| Past Year Suicidal Thoughts (18 or Older) |
0.83 |
0.24 |
0.86 |
0.39 |
1.04 |
0.55 |
| Past Year Suicide Plans (18 or Older) |
0.80 |
0.64 |
0.85 |
0.72 |
1.11 |
0.43 |
| Past Year Suicide Attempts (18 or Older) |
0.81 |
0.71 |
1.15 |
0.80 |
1.40 |
0.08 |
| Past Year Major Depressive Episode (18 or Older) |
0.02 |
0.74 |
0.05 |
1.05 |
0.36 |
COVID-19 = coronavirus disease 2019.
a Significantly different from 1.0 at the .05 level.
Source: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2020. |
References
Ahmad, F. B., Rossen, L. M., & Sutton, P. (2021). Provisional drug overdose death counts. Hyattsville, MD: National Center for Health Statistics. Retrieved on April 13, 2021, from https://www.cdc.gov/nchs/nvss/
Aknin, L., Zaki, J., & Dunn, E. (2021, July 4). The pandemic did not affect mental health the way you think. The Atlantic. Retrieved from https://www.theatlantic.com/ideas/archive/2021/07/covid-19-did-not-affect-mental-health-way-you-think/619354/ 
American Association for Public Opinion Research. (2016). Standard definitions: Final dispositions of case codes and outcome rates for surveys (9th ed. revised). Retrieved from https://www.aapor.org/AAPOR_Main/media/publications/Standard-Definitions20169theditionfinal.pdf 
American Psychiatric Association. (1980). Diagnostic and statistical manual of mental disorders (DSM-III) (3rd ed.). Washington, DC: Author.
American Psychiatric Association. (1987). Diagnostic and statistical manual of mental disorders (DSM-III-R) (3rd rev. ed.). Washington, DC: Author.
American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (DSM-IV) (4th ed.). Washington, DC: Author.
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5) (5th ed.). Arlington, VA: Author.
American Psychological Association. (2020). Stress in America™ 2020: A national mental health crisis. Retrieved from https://www.apa.org/news/press/releases/stress/2020/report-october 
Aquilino, W. S. (1994). Interview mode effects in surveys of drug and alcohol use: A field experiment. Public Opinion Quarterly, 58, 210-240. https://doi.org/10.1086/269419 
Auerbach, R. P., Stewart, J. G., & Johnson, S. L. (2017). Impulsivity and suicidality in adolescent inpatients. Journal of Abnormal Child Psychology, 45(1), 91-103. https://doi.org/10.1007/s10802-016-0146-8 
Bachhuber, M. A., Hennessy, S., Cunningham, C. O., & Starrels, J. L. (2016). Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996-2013. American Journal of Public Health, 106, 686-688. https://doi.org/10.2105/ajph.2016.303061 
Bachman, J. G., Johnston, L. D., O’Malley, P. M., Schulenberg, J. E., & Miech, R. A. (2015). The Monitoring the Future project after four decades: Design and procedures (Monitoring the Future Occasional Paper No. 82). Ann Arbor, MI: Institute for Social Research. Retrieved from http://www.monitoringthefuture.org/pubs/occpapers/mtf-occ82.pdf 
Baker, N. L., Gray, K. M., Sherman, B. J., Morella, K., Sahlem, G. L., Wagner, A. M., & McRae-Clark, A. L. (2018). Biological correlates of self-reported new and continued abstinence in cannabis cessation treatment clinical trials. Drug and Alcohol Dependence, 187, 270-277. https://doi.org/10.1016/j.drugalcdep.2018.03.017 
Barbosa, C., Cowell, A. J., & Dowd, W. N. (2020). Alcohol consumption in response to the COVID-19 pandemic in the United States. Journal of Addiction Medicine. https://doi.org/10.1097/ADM.0000000000000767 
Bassuk, E. L., Richard, M. K., & Tsertsvadze, A. (2015). The prevalence of mental illness in homeless children: A systematic review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 54, 86-96.e2. https://doi.org/10.1016/j.jaac.2014.11.008 
Batts, K., Pemberton, M., Bose, J., Weimer, B., Henderson, L., Penne, M., Gfroerer, J., Trunzo, D., & Strashny, A. (2014, April). Comparing and evaluating substance use treatment utilization estimates from the National Survey on Drug Use and Health and other data sources. CBHSQ Data Review. Retrieved from https://www.samhsa.gov/data/
Beck, A. J., Berzofsky, M., Caspar, R., & Krebs, C. (2013, May). Sexual victimization in prisons and jails reported by inmates, 2011-12: National Inmate Survey, 2011-12 (NCJ 241399). Washington, DC: U.S. Department of Justice, Office of Justice Programs, Bureau of Justice Statistics.
Bray, R. M., Pemberton, M. R., Hourani, L. L., Witt, M., Rae Olmsted, K. L., Brown, J. M., Weimer, B. J., Lane, M. E., Marsden, M. E., Scheffler, S. A., Vandermaas-Peeler, R., Aspinwall, K. R., Anderson, E. M., Spagnola, K., Close, K. L., Gratton, J. L., Calvin, S. L., & Bradshaw, M. R. (2009, September). 2008 Department of Defense Survey of Health Related Behaviors Among Active Duty Military Personnel: A component of the Defense Lifestyle Assessment Program (DLAP) (RTI/10940-FR, prepared under Contract No. GS-10F-0097L, Task Order No. W81XWH-07-F-0538, for the Assistant Secretary of Defense [Health Affairs] and Task Order No. HSCG23-07-F-PMD047 for the U.S. Coast Guard). Research Triangle Park, NC: RTI International.
Brener, N. D., Eaton, D. K., Kann, L., Grunbaum, J. A., Gross, L. A., Kyle, T. M., & Ross, J. G. (2006). The association of survey setting and mode with self-reported health risk behaviors among high school students. Public Opinion Quarterly, 70, 354-374. https://doi.org/10.1093/poq/nfl003 
Bronson, J., & Berzofsky, M. (2017, June). Indicators of mental health problems reported by prisoners and jail inmates, 2011-12 (NCJ 250612, BJS Special Report). Washington, DC: U.S. Department of Justice, Office of Justice Programs, Bureau of Justice Statistics. Retrieved from https://www.bjs.gov/
Bronson, J., Stroop, J., Zimmer, S., & Berzofsky, M. (2017, June). Drug use, dependence, and abuse among state prisoners and jail inmates, 2007-2009 (NCJ 250546, BJS Special Report). Washington, DC: U.S. Department of Justice, Office of Justice Programs, Bureau of Justice Statistics. Retrieved from https://www.bjs.gov/
Buttram, M. E. (2018, March 14). Gabapentin diversion and misuse: Data from law enforcement and substance users. Retrieved from https://www.youtube.com/watch?v=TQK2vn2VjDo 
Buttram, M. E., Kurtz, S. P., Dart, R. C., & Margolin, Z. R. (2017). Law enforcement-derived data on gabapentin research and misuse, 2002-2015: Diversion rates and qualitative research findings. Pharmacoepidemiology and Drug Safety, 26, 1083-1086. https://doi.org/10.1002/pds.4230 
Center for Behavioral Health Statistics and Quality. (2010). Results from the 2009 National Survey on Drug Use and Health: Mental health findings (HHS Publication No. SMA 10-4609, NSDUH Series H-39). Rockville, MD: Substance Abuse and Mental Health Services Administration.
Center for Behavioral Health Statistics and Quality. (2012a). 2010 National Survey on Drug Use and Health: Methodological resource book (Section 16b, Analysis of effects of 2008 NSDUH questionnaire changes: Methods to adjust adult MDE and SPD estimates and to estimate SMI in the 2005-2009 surveys). Rockville, MD: Substance Abuse and Mental Health Services Administration.
Center for Behavioral Health Statistics and Quality. (2012b). Comparing and evaluating youth substance use estimates from the National Survey on Drug Use and Health and other surveys (HHS Publication No. SMA 12-4727, Methodology Series M-9). Rockville, MD: Substance Abuse and Mental Health Services Administration.
Center for Behavioral Health Statistics and Quality. (2012c). Results from the 2011 National Survey on Drug Use and Health: Mental health findings (HHS Publication No. SMA 12-4725, NSDUH Series H-45). Rockville, MD: Substance Abuse and Mental Health Services Administration.
Center for Behavioral Health Statistics and Quality. (2012d). Results from the 2011 National Survey on Drug Use and Health: Summary of national findings (HHS Publication No. SMA 12-4713, NSDUH Series H-44). Rockville, MD: Substance Abuse and Mental Health Services Administration.
Center for Behavioral Health Statistics and Quality. (2013a). 2012 National Survey on Drug Use and Health: Methodological resource book (Section 2, Sample design report). Rockville, MD: Substance Abuse and Mental Health Services Administration.
Center for Behavioral Health Statistics and Quality. (2013b). Results from the 2012 National Survey on Drug Use and Health: Mental health findings (HHS Publication No. SMA 13-4805, NSDUH Series H-47). Rockville, MD: Substance Abuse and Mental Health Services Administration.
Center for Behavioral Health Statistics and Quality. (2014a). 2012 National Survey on Drug Use and Health: Methodological resource book (Section 16a, 2012 Mental Health Surveillance Study: Design and estimation report). Rockville, MD: Substance Abuse and Mental Health Services Administration.
Center for Behavioral Health Statistics and Quality. (2014b). National Survey on Drug Use and Health: 2012 Questionnaire Field Test final report. Rockville, MD: Substance Abuse and Mental Health Services Administration.
Center for Behavioral Health Statistics and Quality. (2014c). National Survey on Drug Use and Health: 2013 Dress Rehearsal final report. Rockville, MD: Substance Abuse and Mental Health Services Administration.
Center for Behavioral Health Statistics and Quality. (2014d). Results from the 2013 National Survey on Drug Use and Health: Summary of national findings (HHS Publication No. SMA 14-4863, NSDUH Series H-48). Rockville, MD: Substance Abuse and Mental Health Services Administration.
Center for Behavioral Health Statistics and Quality. (2015a). 2014 National Survey on Drug Use and Health: Detailed tables. Retrieved from https://www.samhsa.gov/data/
Center for Behavioral Health Statistics and Quality. (2015b). Estimating mental illness among adults in the United States: Revisions to the 2008 estimation procedures. Rockville, MD: Substance Abuse and Mental Health Services Administration.
Center for Behavioral Health Statistics and Quality. (2016). 2015 National Survey on Drug Use and Health: Methodological summary and definitions. Retrieved from https://www.samhsa.gov/data/
Center for Behavioral Health Statistics and Quality. (2017a). 2016 National Survey on Drug Use and Health: Methodological summary and definitions. Retrieved from https://www.samhsa.gov/data/
Center for Behavioral Health Statistics and Quality. (2017b). Evaluation of imputation methods for the National Survey on Drug Use and Health. Retrieved from https://www.samhsa.gov/data/
Center for Behavioral Health Statistics and Quality. (2018a). 2017 National Survey on Drug Use and Health: Detailed tables. Retrieved from https://www.samhsa.gov/data/
Center for Behavioral Health Statistics and Quality. (2018b). 2017 National Survey on Drug Use and Health: Methodological summary and definitions. Retrieved from https://www.samhsa.gov/data/
Center for Behavioral Health Statistics and Quality. (2019). National Survey on Drug Use and Health: 2018 public use file and codebook. Retrieved from https://datafiles.samhsa.gov/
Center for Behavioral Health Statistics and Quality. (2020a). 2019 National Survey on Drug Use and Health: Detailed tables. Retrieved from https://www.samhsa.gov/data/
Center for Behavioral Health Statistics and Quality. (2020b). 2019 National Survey on Drug Use and Health (NSDUH): Methodological resource book (Section 8, Data collection final report). Retrieved from https://www.samhsa.gov/data/
Center for Behavioral Health Statistics and Quality. (2020c). 2019 National Survey on Drug Use and Health (NSDUH): Methodological summary and definitions. Retrieved from https://www.samhsa.gov/data/
Center for Behavioral Health Statistics and Quality. (2020d). 2020 National Survey on Drug Use and Health: Prescription drug images for the 2020 questionnaire. Retrieved from https://www.samhsa.gov/data/
Center for Behavioral Health Statistics and Quality. (2020e). Key substance use and mental health indicators in the United States: Results from the 2019 National Survey on Drug Use and Health (HHS Publication No. PEP20-07-01-001, NSDUH Series H-55). Retrieved from https://www.samhsa.gov/data/
Center for Behavioral Health Statistics and Quality. (2020f). National Survey of Substance Abuse Treatment Services (N-SSATS): 2019. Data on substance abuse treatment facilities. Retrieved from https://www.samhsa.gov/data/
Center for Behavioral Health Statistics and Quality. (2020g). Treatment Episode Data Set (TEDS): 2018. Admissions to and discharges from publicly funded substance use treatment. Retrieved from https://www.samhsa.gov/data/
Center for Behavioral Health Statistics and Quality. (2021a). 2019 National Survey on Drug Use and Health methodological resource book. Retrieved from https://www.samhsa.gov/data/
Center for Behavioral Health Statistics and Quality. (2021b). 2019 National Survey on Drug Use and Health methodological resource book, Section 10: Editing and imputation report. Retrieved from https://www.samhsa.gov/data/
Center for Behavioral Health Statistics and Quality. (2021c). 2019 National Survey on Drug Use and Health: Methodological resource book (Section 11, Person-level sampling weight calibration). Retrieved from https://www.samhsa.gov/data/
Center for Behavioral Health Statistics and Quality. (2021d). 2019 National Survey on Drug Use and Health: Methodological resource book, Section 13: Statistical inference report. Retrieved from https://www.samhsa.gov/data/
Center for Behavioral Health Statistics and Quality. (2021e). 2020 National Survey on Drug Use and Health: Detailed tables. Retrieved from https://www.samhsa.gov/data/
Center for Behavioral Health Statistics and Quality. (2021f). 2020 National Survey on Drug Use and Health (NSDUH) methodological resource book, Section 2: Sample design report. Rockville, MD: Substance Abuse and Mental Health Services Administration.
Center for Behavioral Health Statistics and Quality. (2021g). 2020 National Survey on Drug Use and Health (NSDUH) methodological resource book, Section 14: Sample experience report. Rockville, MD: Substance Abuse and Mental Health Services Administration.
Center for Behavioral Health Statistics and Quality. (2021h). Key substance use and mental health indicators in the United States: Results from the 2020 National Survey on Drug Use and Health (HHS Publication No. PEP21-07-01-003, NSDUH Series H-56). Retrieved from https://www.samhsa.gov/data/
Center for Behavioral Health Statistics and Quality. (forthcoming a). 2020 National Survey on Drug Use and Health methodological resource book. Rockville, MD: Substance Abuse and Mental Health Services Administration.
Center for Behavioral Health Statistics and Quality. (forthcoming b). 2020 National Survey on Drug Use and Health (NSDUH) methodological resource book, Section 8: Data collection final report). Rockville, MD: Substance Abuse and Mental Health Services Administration.
Center for Behavioral Health Statistics and Quality. (forthcoming c). 2020 National Survey on Drug Use and Health (NSDUH) methodological resource book, Section 11: Person-level sampling weight calibration. Rockville, MD: Substance Abuse and Mental Health Services Administration.
Center for Mental Health Services, Substance Abuse and Mental Health Services Administration. (1999, June 24). Estimation methodology for adults with serious mental illness (SMI): Final notice. Federal Register, 64(121), 33890-33897.
Centers for Disease Control and Prevention. (n.d.). COVID Data Tracker: Trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory. Retrieved on June 18, 2021, from https://covid.cdc.gov/covid-data-tracker/#trends_dailytrendscases
Centers for Disease Control and Prevention. (2013, March 29). QuickStats: Number of deaths from poisoning, drug poisoning, and drug poisoning involving opioid analgesics—United States, 1999-2010. Morbidity and Mortality Weekly Report, 62(12), 234.
Centers for Disease Control and Prevention. (2019). BRFSS prevalence & trends data. Retrieved from https://www.cdc.gov/brfss/brfssprevalence/index.html
Cohen, R. I. S., & Bosk, E. A. (2020). Vulnerable youth and the COVID-19 pandemic. Pediatrics, 146(1), e20201306. https://doi.org/10.1542/peds.2020-1306 
Colpe, L. J., Epstein, J. F., Barker, P. R., & Gfroerer, J. C. (2009). Screening for serious mental illness in the National Survey on Drug Use and Health (NSDUH). Annals of Epidemiology, 19, 210-211. https://doi.org/10.1016/j.annepidem.2008.09.005 
Controlled Substances Act, 21 U.S.C., §§ 801-971. (2012). Retrieved from https://www.deadiversion.usdoj.gov/21cfr/21usc/index.html
Czeisler, M. É., Lane, R. I., Petrosky E., Wiley, J. F., Christensen, A., Njai, R., Weaver, M. D., Robbins, R., Facer-Childs, E. R., Barger, L. K., Czeisler, C. A., Howard, M. E., & Rajaratnam, S. M. W. (2020). Mental health, substance use, and suicidal ideation during the COVID-19 pandemic — United States, June 24-30, 2020. Morbidity Mortality Weekly Report, 69(32), 1049-1057. Retrieved from https://www.cdc.gov/mmwr/volumes/69/wr/mm6932a1.htm?s_cid=mm6932a1_w
Daikeler, J., Bošnjak, M., & Manfreda, K. L. (2020). Web versus other survey modes: An updated and extended meta-analysis comparing response rates. Journal of Survey Statistics and Methodology, 8(3), 513-539.
Daly, M., & Robinson, E. (2021). Psychological distress and adaptation to the COVID-19 crisis in the United States. Journal of Psychiatric Research, 136, 603-609. https://doi.org/10.1016/j.jpsychires.2020.10.035 
Deisenhammer, E. A., Ing, C. M., Strauss, R., Kemmler, G., Hinterhuber, H., & Weiss, E. M. (2009). The duration of the suicidal process: How much time is left for intervention between consideration and accomplishment of a suicide attempt? Journal of Clinical Psychiatry, 70(1), 19-24.
Deville, J.-C., & Särndal, C.-E. (1992). Calibration estimators in survey sampling. Journal of the American Statistical Association, 87, 376-382. https://doi.org/10.1080/01621459.1992.10475217 
Dowell, D., Haegerich, T. M., & Chou, R. (2016). CDC guideline for prescribing opioids for chronic pain — United States, 2016. Morbidity and Mortality Weekly Report Recommendations and Reports, 65(RR-1), 1-50. Retrieved from https://www.cdc.gov/mmwr/volumes/65/rr/pdfs/rr6501e1.pdf [Errata]. Vol. 65, No. RR-1. 2016. Morbidity and Mortality Weekly Report, 65, 295. https://doi.org/10.15585/mmwr.mm6511a6 
Endicott, J., Spitzer, R. L., Fleiss, J. L., & Cohen, J. (1976). The Global Assessment Scale: A procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry, 33, 766-771. https://doi.org/10.1001/archpsyc.1976.01770060086012 
European Monitoring Centre for Drugs and Drug Addiction. (2021). European Drug Report 2021: Trends and developments. Luxembourg: Publications Office of the European Union.
Fagerstrom, K.-O. (1978). Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addictive Behaviors, 3, 235-241. https://doi.org/10.1016/0306-4603(78)90024-2 
Fendrich, M., Johnson, T. P., Sudman, S., Wislar, J. S., & Spiehler, V. (1999). Validity of drug use reporting in a high-risk community sample: A comparison of cocaine and heroin survey reports with hair tests. American Journal of Epidemiology, 149, 955-962. https://doi.org/10.1093/oxfordjournals.aje.a009740 
First, M. B., Spitzer, R. L., Gibbon, M., & Williams, J. B. W. (2002). Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP). New York, NY: New York State Psychiatric Institute, Biometrics Research.
First, M. B., Williams, J. B. W., Karg, R. S., & Spitzer, R. L. (2015). Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV). Arlington, VA: American Psychiatric Association.
Folsom, R. E., & Singh, A. C. (2000). The generalized exponential model for sampling weight calibration for extreme values, nonresponse, and poststratification. In Proceedings of the 2000 Joint Statistical Meetings, American Statistical Association, Survey Research Methods Section, Indianapolis, IN (pp. 598-603). Alexandria, VA: American Statistical Association.
Fowler, F. J., & Stringfellow, V. L. (2001). Learning from experience: Estimating teen use of alcohol, cigarettes, and marijuana from three survey protocols. Journal of Drug Issues, 31, 643-664. https://doi.org/10.1177/002204260103100304 
Gentzke, A. S., Wang, T. W., Jamal, A., Park-Lee, E., Ren, C., Cullen, K. A., & Neff, L. (2020). Tobacco product use among middle and high school students — United States, 2020. Morbidity and Mortality Weekly Report, 69, 1882-1888. https://doi.org/10.15585/mmwr.mm6950a1 
Gfroerer, J., Eyerman, J., & Chromy, J. (Eds.). (2002). Redesigning an ongoing national household survey: Methodological issues (HHS Publication No. SMA 03-3768). Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies.
Gfroerer, J., Lessler, J., & Parsley, T. (1997a). Studies of nonresponse and measurement error in the National Household Survey on Drug Abuse. In L. Harrison & A. Hughes (Eds.), The validity of self-reported drug use: Improving the accuracy of survey estimates (NIH Publication No. 97-4147, NIDA Research Monograph 167, pp. 273-295). Rockville, MD: National Institute on Drug Abuse.
Gfroerer, J., Wright, D., & Kopstein, A. (1997b). Prevalence of youth substance use: The impact of methodological differences between two national surveys. Drug and Alcohol Dependence, 47, 19-30. https://doi.org/10.1016/s0376-8716(97)00063-x 
Glasheen, C., Hedden, S. L., Kroutil, L. A., Pemberton, M. R., & Goldstrum, I. (2012, November). Past year arrest among adults in the United States: Characteristics of and association with mental illness and substance use. CBHSQ Data Review. Retrieved from https://www.samhsa.gov/data/
Grant, B. F., & Dawson, D. A. (2006). Introduction to the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Research & Health, 29, 74-78.
Grant, B. F., Goldstein, R. B., Saha, T. D., Chou, S. P., Jung, J., Zhang, H., Pickering, R. P., Ruan, W. J., Smith, S. M., Huang, B., & Hasin, D. S. (2015). Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. Journal of the American Medical Association Psychiatry, 72, 757-766. https://doi.org/10.1001/jamapsychiatry.2015.0584 
Grant, B. F., Stinson, F. S., Dawson, D. A., Chou, S. P., Dufour, M. C., Compton, W., Pickering, R. P., & Kaplan, K. (2004). Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry, 61, 807-816. https://doi.org/10.1001/archpsyc.61.8.807 
Grossman, E. R., Benjamin-Neelon, S. E., & Sonnenschein, S. (2020). Alcohol consumption during the COVID-19 pandemic: A cross-sectional survey of US adults. International Journal of Environmental Research and Public Health, 17(24), 9189. https://doi.org/10.3390/ijerph17249189 
Gunderson, J., Mitchell, D., Reid, K., & Jordan, M. (2021). COVID-19 information-seeking and prevention behaviors in Florida, April 2020. Preventing Chronic Disease, 18. https://doi.org/10.5888/pcd18.200575 
Haberstick, B. C., Young, S. E., Zeiger, J. S., Lessem, J. M., Hewitt, J. K., & Hopfer, C. J. (2014). Prevalence and correlates of alcohol and cannabis use disorders in the United States: Results from the National Longitudinal Study of Adolescent Health. Drug and Alcohol Dependence, 136, 158-161. https://doi.org/10.1016/j.drugalcdep.2013.11.022 
Harris, K. M., Halpern, C. T., Biemer, P., Liao, D., & Dean, S. C. (2019). Add Health Wave V documentation: Sampling and mixed-mode survey design. Retrieved from http://www.cpc.unc.edu/projects/addhealth/documentation/guides/ 
Harrison, L. D. (2001). Understanding the differences in youth drug prevalence rates produced by the MTF, NHSDA, and YRBS studies. Journal of Drug Issues, 31, 665-694. https://doi.org/10.1177/002204260103100305 
Harrison, L., & Hughes, A. (Eds.). (1997). The validity of self-reported drug use: Improving the accuracy of survey estimates (NIH Publication No. 97-4147, NIDA Research Monograph 167). Rockville, MD: National Institute on Drug Abuse.
Harrison, L. D., Martin, S. S., Enev, T., & Harrington, D. (2007). Comparing drug testing and self-report of drug use among youths and young adults in the general population (HHS Publication No. SMA 07-4249, Methodology Series M-7). Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies.
Hasin, D. S., Greenstein, E., Aivadyan, C., Stohl, M., Aharonovich, E., Saha, T., Goldstein, R., Nunes, E. V., Jung, J., Zhang, H., & Grant, B. F. (2015). The Alcohol Use Disorder and Associated Disabilities Interview Schedule-5 (AUDADIS-5): Procedural validity of substance use disorders modules through clinical re-appraisal in a general population sample. Drug and Alcohol Dependence, 148, 40-46. https://doi.org/10.1016/j.drugalcdep.2014.12.011 
Havens, J. R. (2018, March 14). The emergence of gabapentin as a drug of abuse in a cohort of rural Appalachian drug users. Retrieved from https://www.youtube.com/watch?v=TQK2vn2VjDo 
Heatherton, T. F., Kozlowski, L. T., Frecker, R. C., & Fagerstrom, K.-O. (1991). The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction, 86, 1119-1127. https://doi.org/10.1111/j.1360-0443.1991.tb01879.x 
Hedden, S., Gfroerer, J., Barker, P., Smith, S., Pemberton, M. R., Saavedra, L. M., Forman-Hoffman, V. L., Ringeisen, H., & Novak, S. P. (2012, March). Comparison of NSDUH mental health data and methods with other data sources. CBHSQ Data Review. Retrieved from https://www.samhsa.gov/data/
Hedegaard, H., Bastian, B. A., Trinidad, J. P., Spencer, M. R., & Warner, M. (2019, October 25). Regional differences in drugs most frequently involved in drug overdose deaths: United States, 2017 (National Vital Statistics Reports, Vol. 68, No. 12). Retrieved from https://www.cdc.gov/nchs/data/nvsr/nvsr68/nvsr68_12-508.pdf
Hedegaard, H., Miniño, A. M., & Warner, M. (2020, December). Drug overdose deaths in the United States, 1999-2019. NCHS Data Brief, no 394. Hyattsville, MD: National Center for Health Statistics. Retrieved from https://www.cdc.gov/nchs/data/databriefs/db394-H.pdf
Hossain, M. M., Tasnim, S., Sultana, A., Faizah, F., Mazumder, H., Zou, L., McKyer, E. L. J., Ahmed, H. U., & Ma, P. (2020). Epidemiology of mental health problems in COVID-19: A review. F1000 Research, 9, 636. https://doi.org/10.12688/f1000research.24457.1 
Hughes, A., Williams, M. R., Lipari, R. N., Bose, J., Copello, E. A. P., & Kroutil, L. A. (2016, September). Prescription drug use and misuse in the United States: Results from the 2015 National Survey on Drug Use and Health. NSDUH Data Review. Retrieved from https://www.samhsa.gov/data/
Humensky, J. L. (2010). Are adolescents with high socioeconomic status more likely to engage in alcohol and illicit drug use in early adulthood? Substance Abuse Treatment, Prevention, and Policy, 5, 19. https://doi.org/10.1186/1747-597x-5-19 
Interagency Council on Statistical Policy. (2021, January 15). Statistical agency changes in response to the COVID-19 pandemic. Retrieved on July 15, 2021, from https://www.bea.gov/system/files/2021-01/ICSP-COVID-19-Report_011521.pdf
Johnston, L. D., Miech, R. A., O’Malley, P. M., Bachman, J. G., Schulenberg, J. E., & Patrick, M. E. (2020). Monitoring the Future national survey results on drug use, 1975-2019: 2019 overview, key findings on adolescent drug use. Ann Arbor, MI: University of Michigan, Institute for Social Research. Retrieved from http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2019.pdf 
Kessler, R. C., Avenevoli, S., Costello, J., Green, J. G., Gruber, M. J., Heeringa, S., Merikangas, K. R., Pennell, B. E., Sampson, N. A., & Zaslavsky, A. M. (2009). Design and field procedures in the US National Comorbidity Survey Replication Adolescent Supplement (NCS-A). International Journal of Methods in Psychiatric Research, 18(2), 69-83. https://doi.org/10.1002/mpr.279 
Kessler, R. C., Barker, P. R., Colpe, L. J., Epstein, J. F., Gfroerer, J. C., Hiripi, E., Howes, M. J., Normand, S. L., Manderscheid, R. W., Walters, E. E., & Zaslavsky, A. M. (2003a). Screening for serious mental illness in the general population. Archives of General Psychiatry, 60, 184-189. https://doi.org/10.1001/archpsyc.60.2.184 
Kessler, R. C., Berglund, P. A., Bruce, M. L., Koch, J. R., Laska, E. M., Leaf, P. J., Manderscheid, R. W., Rosenheck, R. A., Walters, E. E., & Wang, P. S. (2001). The prevalence and correlates of untreated serious mental illness. Health Services Research, 36, 987-1007.
Kessler, R. C., Berglund, P., Chiu, W. T., Demler, O., Heeringa, S., Hiripi, E., Jin, R., Pennell, B. E., Walters, E. E., Zaslavsky, A, & Zheng, H. (2004a). The US National Comorbidity Survey Replication (NCS-R): Design and field procedures. International Journal of Methods in Psychiatric Research, 13(2), 69-92. https://doi.org/10.1002/mpr.167 
Kessler, R. C., Berglund, P., Demler, O., Jin, R., Koretz, D., Merikangas, K. R., Rush, A. J., Walters, E. E., & Wang, P. S. (2003b). The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R). Journal of the American Medical Association, 289, 3095-3105. https://doi.org/10.1001/jama.289.23.3095 
Kessler, R. C., Berglund, P. A., Glantz, M. D., Koretz, D. S., Merikangas, K. R., Walters, E. E., & Zaslavsky, A. M. (2004b). Estimating the prevalence and correlates of serious mental illness in community epidemiological surveys. In R. W. Manderscheid & M. J. Henderson (Eds.), Mental health, United States, 2002 (HHS Publication No. SMA 04-3938, pp. 155-164). Rockville, MD: Substance Abuse and Mental Health Services Administration, Center for Mental Health Services.
Kessler, R. C., Berglund, P. A., Walters, E. E., Leaf, P. J., Kouzis, A. C., Bruce, M. L., Friedman, R. M., Grosser, R. C., Kennedy, C., Kuehnel, T. G., Laska, E. M., Manderscheid, R. W., Narrow, W. E., Rosenheck, R. A., & Schneier, M. (1998). A methodology for estimating the 12-month prevalence of serious mental illness. In R. W. Manderscheid & M. J. Henderson (Eds.), Mental health, United States, 1998 (HHS Publication No. SMA 99-3285, pp. 99-109). Rockville, MD: Substance Abuse and Mental Health Services Administration, Center for Mental Health Services.
Kessler, R. C., Berglund, P. A., Zhao, S., Leaf, P. J., Kouzis, A. C., Bruce, M. L., Friedman, R. M., Grosser, R. C., Kennedy, C., Narrow, W. E., Kuehnel, T. G., Laska, E. M., Manderscheid, R. W., Rosenheck, R. A., Santoni, T. W., & Schneier, M. (1996). The 12-month prevalence and correlates of serious mental illness (SMI). In R. W. Manderscheid & M. A. Sonnenschein (Eds.), Mental health, United States, 1996 (HHS Publication No. SMA 96-3098, pp. 59-70). Rockville, MD: Substance Abuse and Mental Health Services Administration.
Kessler, R. C., Chiu, W. T., Colpe, L., Demler, O., Merikangas, K. R., Walters, E. E., & Wang, P. S. (2006). The prevalence and correlates of serious mental illness (SMI) in the National Comorbidity Survey Replication (NCS-R). In R. W. Manderscheid & J. T. Berry (Eds.), Mental health, United States, 2004 (pp. 134-148). Rockville, MD: Substance Abuse and Mental Health Services Administration.
Kessler, R. C., Colpe, L. J., Fullerton, C. S., Gebler, N., Naifeh, J. A., Nock, M. K., Sampson, N. A., Schoenbaum, M., Zaslavsky, A. M., Stein, M. B., Ursano, R. J., & Herringa, S. G. (2013). Design of the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). International Journal of Methods in Psychiatric Research, 22, 267-275. https://doi.org/10.1002/mpr.1401 
Kessler, R. C., Herringa, S. G., Stein, M. B., Colpe, L. J., Fullerton, C. S., Hwang, I., Naifeh, J. A., Nock, M. K., Petukhova, M., Sampson, N. A., Schoenbaum, M., Zaslavsky, A. M., & Ursano, R. J. (2014). Thirty-day prevalence of DSM-IV mental disorders among nondeployed soldiers in the US Army: Results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). Journal of the American Medical Association Psychiatry, 71(5), 504-513. https://doi.org/10.1001/jamapsychiatry.2014.28 
Kessler, R. C., & Üstün, T. B. (2004). The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). International Journal of Methods in Psychiatric Research, 13, 93-121. https://doi.org/10.1002/mpr.168 
Klonsky, E. D., & May, A. (2010). Rethinking impulsivity in suicide. Suicide and Life-Threatening Behavior, 40(6), 612-619.
Kreuter, F., Presser, S., & Tourangeau, R. (2008). Social desirability bias in CAI, IVR, and web surveys: The effects of mode and question sensitivity. Public Opinion Quarterly, 72, 847-865. https://doi.org/10.1093/poq/nfn063 
Lattimore, P. K., Steffey, D. M., Gfroerer, J., Bose, J., Pemberton, M. R., & Penne, M. A. (2014, August). Arrestee substance use: Comparison of estimates from the National Survey on Drug Use and Health and the Arrestee Drug Abuse Monitoring Program. CBHSQ Data Review. Retrieved from https://www.samhsa.gov/data/
Leon, A. C., Olfson, M., Portera, L., Farber, L., & Sheehan, D. V. (1997). Assessing psychiatric impairment in primary care with the Sheehan Disability Scale. International Journal of Psychiatry in Medicine, 27(2), 93-105. https://doi.org/10.2190/t8em-c8yh-373n-1uwd 
Lerner, G., Daly, D., & Anderson, M. (2021). Effects of severe weather and COVID-19 pandemic on survey response rates and survey methods for the Behavioral Risk Factor Surveillance System (BRFSS). Presented at the 2021 American Association for Public Opinion Research Annual Conference. Retrieved on June 8, 2021, from https://aapor.secure-platform.com/a/solicitations/12/sessiongallery/571/application/4811 
Li, D. H., Janulis, P., & Mustanski, B. (2019). Predictors of correspondence between self-reported substance use and urinalysis screening among a racially diverse cohort of young men who have sex with men and transgender women. Addictive Behaviors, 88, 6-14. https://doi.org/10.1016/j.addbeh.2018.08.004 
Lind, L., Schober, M., Conrad, F., & Reichert, H. (2013). Why do survey respondents disclose more when computers ask the questions? Public Opinion Quarterly, 77, 888-935.
Lindberg, L., & Scott, R. H. (2018). Effect of ACASI on reporting of abortion and other pregnancy outcomes in the US National Survey of Family Growth. Studies in Family Planning, 49, 259-278. https://doi.org/10.1111/sifp.12068 
Lofquist, D., Lugaila, T., O’Connell, M., & Feliz, S. (2012, April). Households and families: 2010 (C2010BR-14, 2010 Census Briefs). Retrieved from https://www.census.gov/prod/cen2010/briefs/c2010br-14.pdf
Lozar-Manfreda, K., & Vehovar, V. (2002). Survey design features influencing response rates in web surveys. Ljubljana, Slovenia: University of Ljubljana.
Manly, B. F. J. (1986). Multivariate statistical methods: A primer. London, England: Chapman and Hall.
Mavletova, A., & Couper, M. P. (2015). A meta-analysis of breakoff rates in mobile web surveys. In D. Toninelli, R. Pinter, & P. de Pedraza (Eds.), Mobile research methods: Opportunities and challenges of mobile research methodologies (pp. 81-98). London: Ubiquity Press.
Meadows, S. O., Engel, C. C., Collins, R. L., Beckman, R., Cefalu, M., Hawes-Dawson, J., Doyle, M., Kress, A. M., Sontag-Padilla, L., Ramchand, R., & Williams, K. M. (2018). 2015 Department of Defense Health Related Behaviors Survey (HRBS) (prepared for the Defense Health Agency). Santa Monica, CA: Rand Corporation. Retrieved from https://www.health.mil/Military-Health-Topics/Access-Cost-Quality-and-Safety/Health-Care-Program-Evaluation/Survey-of-Health-Related-Behaviors/2015-Health-Related-Behavior-Survey-Active-Duty 
Miech, R. A., Johnston, L. D., O’Malley, P. M., Bachman, J. G., Schulenberg, J. E., & Patrick, M. E. (2017). Monitoring the Future national survey results on drug use, 1975-2016: Volume I, Secondary school students. Ann Arbor, MI: University of Michigan Institute for Social Research. Retrieved from http://www.monitoringthefuture.org/pubs/monographs/mtf-vol1_2016.pdf 
Miech, R. A., Johnston, L. D., O’Malley, P. M., Bachman, J. G., Schulenberg, J. E., & Patrick, M. E. (2021). Monitoring the Future national survey results on drug use, 1975-2020: Volume I, Secondary school students. Ann Arbor, MI: University of Michigan Institute for Social Research. Retrieved from http://www.monitoringthefuture.org/pubs/monographs/mtf-vol1_2020.pdf 
Miller, G. K., Piscopo, K. D., Batts, K., Han, B., Colpe, L., Forman-Hoffman, V. L., Gfroerer, J., & McKeon, R. T. (2015, July). Measurement of suicidal thoughts, behaviors, and related health outcomes in the United States: Comparison of NSDUH estimates with other data sources. CBHSQ Data Review. Retrieved from https://www.samhsa.gov/data/
Miller, K. (2019). NCHS opioid question evaluation study. Hyattsville, MD: National Center for Health Statistics. Retrieved on July 16, 2021, from https://www.cdc.gov/nchs/data/bsc/bsc_pres_miller_may_2019.pdf
Miron, O., Yu, K.-H., Wilf-Miron, R., & Kohane, I. S. (2019). Suicide rates among adolescents and young adults in the United States, 2000-2017. JAMA, 321, 2362-2364. https://doi.org/10.1001/jama.2019.5054 
Mojtabai, R., & Olfson, M. (2020). National trends in mental health care for US adolescents. JAMA Psychiatry, 77, 703-714. https://doi.org/10.1001/jamapsychiatry.2020.0279 
Mojtabai, R., Olfson, M., & Han, B. (2016). National trends in the prevalence and treatment of depression in adolescents and young adults. Pediatrics, 138(6), e20161878. https://doi.org/10.1542/peds.2016-1878 
Moreland, A., Herlihy, C., Tynan, M. A., Sunshine, G., McCord, R. F., Hilton, C., Poovey, J., Werner, A. K., Jones, C. D., Fulmer, E. B., Gundlapalli, A. V., Strosnider, H., Potvien, A., García, M. C., Honeycutt, S., Baldwin, G., CDC Public Health Law Program, & CDC COVID-19 Response Team, Mitigation Policy Analysis Unit. (2020). Timing of state and territorial COVID-19 stay-at-home orders and changes in population movement — United States, March 1-May 31, 2020. Morbidity and Mortality Weekly Report, 69, 1198-1203.
Naifeh, J. A., Colpe, L. J., Aliaga, P. A., Sampson, N. A., Heeringa, S. G., Stein, M. B., Ursano, R. J., Fullerton, C. S., Nock, M. K., Schoenbaum, M., Zaslavsky, A. M., & Kessler, R. C. (2016). Barriers to initiating and continuing mental health treatment among soldiers in the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). Military Medicine, 181, 1021-1032. https://doi.org/10.7205/milmed-d-15-00211 
Naifeh, J. A., Ursano, R. J., Kessler, R. C., Gonzalez, O. I., Fullerton, C. S., Herberman Mash, H. B., Riggs-Donovan, C. A., Ng, T. H. H., Wynn, G. H., Dinh, H. M., Kao, T.-C., Sampson, N. A., & Stein, M. B. (2019). Suicide attempts among activated soldiers in the U.S. Army reserve components. BMC Psychiatry, 19(1), 31. https://doi.org/10.1186/s12888-018-1978-2 
Narrow, W. E., Rae, D. S., Robins, L. N., & Regier, D. A. (2002). Revised prevalence estimates of mental disorders in the United States: Using a clinical significance criterion to reconcile 2 surveys’ estimates. Archives of General Psychiatry, 59, 115-123. https://doi.org/10.1001/archpsyc.59.2.115 
National Advisory Mental Health Council. (1993). Health care reform for Americans with severe mental illnesses: Report of the National Advisory Mental Health Council. American Journal of Psychiatry, 150, 1447-1465. https://doi.org/10.1176/ajp.150.10.1447 
National Center for Health Statistics. (n.d.-a). Anxiety and depression. Household Pulse Survey. Retrieved on June 8, 2021, from https://www.cdc.gov/nchs/covid19/pulse/mental-health.htm
National Center for Health Statistics. (n.d.-b). Reduced access to care. Household Pulse Survey. Retrieved on July 8, 2021, from https://www.cdc.gov/nchs/covid19/pulse/reduced-access-to-care.htm
National Center for Health Statistics. (2018a, June). 2017 National Health Interview Survey public use data release: Survey description. Retrieved from https://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NHIS/2017/srvydesc.pdf
National Center for Health Statistics. (2018b, November 30). Table A-12a. Current cigarette smoking status among adults aged 18 and over, by selected characteristics: United States, 2017. In Tables of summary health statistics. Retrieved from https://www.cdc.gov/nchs/nhis/shs/tables.htm
National Center for Health Statistics. (2019). 2017 [National Hospital Ambulatory Medical Care Survey] NHAMCS micro-data file documentation. Retrieved from https://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NHAMCS/doc17_ed-508.pdf
National Center for Health Statistics. (2020). National Health Interview Survey, 2019. Public-use data file and documentation. Retrieved on June 8, 2021, from https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
National Center for Health Statistics. (2021). 2020 National Health Interview Survey: Note to data users about changes in data collection during the 2020 calendar year. Retrieved on June 8, 2021, from https://www.cdc.gov/nchs/nhis/2020nhis.htm
National Institute on Alcohol Abuse and Alcoholism. (2010, September). Alcohol use and alcohol use disorders in the United States, a 3-year follow-up: Main findings from the 2004-2005 Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) (U.S. Alcohol Epidemiologic Data Reference Manual, Vol. 8, No. 2, NIH Publication No. 10-7677). Bethesda, MD: National Institutes of Health.
National Institute on Alcohol Abuse and Alcoholism. (2021). Dataset of state alcohol-related laws during the COVID-19 emergency for on-premises and off-premises establishments as of April 15, 2021. Alcohol Policy Information System (APIS). Retrieved from https://alcoholpolicy.niaaa.nih.gov/resource/covid-19/98
National Institute on Drug Abuse. (2018a, March). DrugFacts: Prescription CNS depressants. Retrieved from https://www.drugabuse.gov/publications/finder/t/160/drugfacts
National Institute on Drug Abuse. (2018b, June). DrugFacts: Prescription stimulants. Retrieved from https://www.drugabuse.gov/publications/finder/t/160/drugfacts
National Institute on Drug Abuse. (2019a, April). DrugFacts: Kratom. Retrieved from https://www.drugabuse.gov/publications/finder/t/160/drugfacts
National Institute on Drug Abuse. (2019b, May). DrugFacts: Methamphetamine. Retrieved from https://www.drugabuse.gov/publications/finder/t/160/drugfacts
National Institute on Drug Abuse. (2020a, May). DrugFacts: Prescription opioids. Retrieved from https://www.drugabuse.gov/publications/finder/t/160/drugfacts
National Institute on Drug Abuse. (2020b, June). DrugFacts: Synthetic cannabinoids (K2/Spice). Retrieved from https://www.drugabuse.gov/publications/finder/t/160/drugfacts
National Institute on Drug Abuse. (2020c, July). DrugFacts: Synthetic cathinones (bath salts). Retrieved from https://www.drugabuse.gov/publications/finder/t/160/drugfacts
National Institute on Drug Abuse. (2021a, January 29). Overdose death rates. Retrieved from https://www.drugabuse.gov
National Institute on Drug Abuse. (2021b, April). DrugFacts: Cocaine. Retrieved from https://www.drugabuse.gov/publications/finder/t/160/drugfacts
Nock, M. K., Stein, M. B., Herringa, S. G., Ursano, R. J., Colpe, L. J., Fullerton, C. S., Hwang, I., Naifeh, J. A., Sampson, N. A., Schoenbaum, M., Zaslavsky, A. M., & Kessler, R. C. (2014). Prevalence and correlates of suicidal behavior among soldiers: Results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). Journal of the American Medical Association Psychiatry, 71, 514-522. https://doi.org/10.1001/jamapsychiatry.2014.30 
Novak, S. (2007, October). An item response analysis of the World Health Organization Disability Assessment Schedule (WHODAS) items in the 2002-2004 NSDUH (prepared for the Substance Abuse and Mental Health Services Administration under Contract No. 283-03-9028, RTI/8726). Research Triangle Park, NC: RTI International.
Novak, S. P., Colpe, L. J., Barker, P. R., & Gfroerer, J. C. (2010). Development of a brief mental health impairment scale using a nationally representative sample in the USA. International Journal of Methods in Psychiatric Research, 19(Suppl. 1), 49-60. https://doi.org/10.1002/mpr.313 
Nurin, T. (2020, June 30). Alcohol sales are not spiking or even stabilizing. Here’s why the misconception matters. Forbes.
Office of Applied Studies. (2005). Results from the 2004 National Survey on Drug Use and Health: National findings (HHS Publication No. SMA 05-4062, NSDUH Series H-28). Rockville, MD: Substance Abuse and Mental Health Services Administration.
Office of Applied Studies. (2009a). 2008 National Survey on Drug Use and Health: Methodological resource book (Section 16, Measuring serious mental illness with the NSDUH: Results of 2008 12-month analysis). Rockville, MD: Substance Abuse and Mental Health Services Administration.
Office of Applied Studies. (2009b). Results from the 2008 National Survey on Drug Use and Health: National findings (HHS Publication No. SMA 09-4434, NSDUH Series H-36). Rockville, MD: Substance Abuse and Mental Health Services Administration.
Palamar, J. J., Le, A., Guarino, H., & Mateu-Gelabert, P. (2019). A comparison of the utility of urine- and hair testing in detecting self-reported drug use among young adult opioid users. Drug and Alcohol Dependence, 200, 161-167. https://doi.org/10.1016/j.drugalcdep.2019.04.008 
Patrick, M. E., Couper, M. P., Parks, M. J., Laetz, V., & Schulenberg, J. E. (2021). Comparison of web-push survey research protocol with the mailed paper and pencil protocol in the Monitoring the Future panel survey. Addiction, 116, 191-199. https://doi.org/10.1111/add.15158 
Paulozzi, L. J. (2012). Prescription drug overdoses: A review. Journal of Safety Research, 43, 283-289. https://doi.org/10.1016/j.jsr.2012.08.009 
Pemberton, M. R., Bose, J., Kilmer, G., Kroutil, L. A., Forman-Hoffman, V. L., & Gfroerer, J. C. (2013, September). Comparison of NSDUH health and health care utilization estimates to other national data sources. CBHSQ Data Review. Retrieved from https://www.samhsa.gov/data/
Perrin, A., & Atske, S. (2021, April 2). 7% of Americans don’t use the internet. Who are they? Pew Research Center. Retrieved from https://www.pewresearch.org/fact-tank/2021/04/02/7-of-americans-dont-use-the-internet-who-are-they/ 
Peytchev, A. (2009). Survey breakoff. Public Opinion Quarterly, 73, 74-97. https://doi.org/10.1093/poq/nfp014 
Pollard, M. S., Tucker, J. S., & Green, Jr., H. D. (2020). Changes in adult alcohol use and consequences during the COVID-19 pandemic in the US. JAMA Network Open, 3(9), e2022942. https://doi.org/10.1001/jamanetworkopen.2020.22942 
Prison Rape Elimination Act (PREA), Pub. L. 108-79 (2003). Retrieved from https://www.govinfo.gov/app/details/PLAW-108publ79
Reiley, L. (2020, May 23). Americans are drinking more during the pandemic. Craft distillers aren’t getting any of the love. The Washington Post.
Rendon, A., Livingston, M., Suzuki, S., Hill, W., & Walters, S. (2017). What’s the agreement between self-reported and biochemical verification of drug use? A look at permanent supportive housing residents. Addictive Behaviors, 70, 90-96. https://doi.org/10.1016/j.addbeh.2017.02.011 
Rendon, A., Mun, E. Y., Spence-Almaguer, E., & Walters, S. T. (2019). What happens to agreement over time? A longitudinal study of self-reported substance use compared to saliva toxicological testing among subsidized housing residents. Journal of Substance Abuse Treatment, 101, 12-17. https://doi.org/10.1016/j.jsat.2019.03.002 
Rodway, C., Tham, S.-G., Turnbull, P., Kapur, N., & Appleby, L. (2020). Suicide in children and young people: Can it happen without warning? Journal of Affective Disorders, 275, 307-310. https://doi.org/10.1016/j.jad.2020.06.069 
Rosellini, A. J., Heeringa, S. G., Stein, M. B., Ursano, R. J., Chiu, W. T., Colpe, L. J., Fullerton, C. S., Gilman, S. E., Hwang, I., Naifeh, J. A., Nock, M. K., Petukhova, M., Sampson, N. A., Schoenbaum, M., Zaslavsky, A. M., & Kessler, R. C. (2015). Lifetime prevalence of DSM-IV mental disorders among new soldiers in the U.S. Army: Results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). Depression and Anxiety, 32(1), 13-24. https://doi.org/10.1002/da.22316 
Rowe, C., Vittinghoff, E., Colfax, G., Coffin, P. O., & Santos, G. M. (2018). Correlates of validity of self-reported methamphetamine use among a sample of dependent adults. Substance Use & Misuse, 53(10), 1742-1755. https://doi.org/10.1080/10826084.2018.1432649 
RTI International. (2013). SUDAAN® language manual, Release 11.0.1. Research Triangle Park, NC: Author.
Rubin, D. B. (1986). Statistical matching using file concatenation with adjusted weights and multiple imputations. Journal of Business and Economic Statistics, 4(1), 87-94. https://doi.org/10.2307/1391390 
Schilling, E. A., Aseltine, R. H, Glanovsky, J. L., James, A., & Jacobs, D. (2009). Adolescent alcohol use, suicidal ideation, and suicide attempts. Journal of Adolescent Health, 44, 335-341. https://doi.org/10.1016/j.jadohealth.2008.08.006 
Schulenberg, J. E., Johnston, L. D., O’Malley, P. M., Bachman, J. G., Miech, R. A. & Patrick, M. E. (2017). Monitoring the Future national survey results on drug use, 1975-2016: Volume II, College students and adults ages 19-55. Ann Arbor, MI: Institute for Social Research, University of Michigan. Retrieved from http://www.monitoringthefuture.org/pubs/monographs/mtf-vol2_2016.pdf 
Schulenberg, J. E., Johnston, L. D., O’Malley, P. M., Bachman, J. G., Miech, R. A., & Patrick, M. E. (2019). Monitoring the Future national survey results on drug use, 1975-2018: Volume II, College students and adults ages 19-60. Ann Arbor: Institute for Social Research, University of Michigan. Retrieved from http://monitoringthefuture.org/pubs/monographs/mtf-vol2_2018.pdf 
Schulenberg, J. E., Johnston, L. D., O’Malley, P. M., Bachman, J. G., Miech, R. A., & Patrick, M. E. (2020). Monitoring the Future national survey results on drug use, 1975-2019: Volume II, College students and adults ages 19-60. Ann Arbor: Institute for Social Research, University of Michigan. Retrieved from http://www.monitoringthefuture.org/pubs/monographs/mtf-vol2_2019.pdf 
Seth, P., Scholl, L., Rudd, R. A., & Bacon, S. (2018). Overdose deaths involving opioids, cocaine, and psychostimulants—United States, 2015-2016. Morbidity and Mortality Weekly Report, 67(12), 349-358. https://doi.org/10.15585/mmwr.mm6712a1 
Shiffman, S., Hickcox, M., Gnys, M., Paty, J. A., & Kassel, J. D. (1995, March). The Nicotine Dependence Syndrome Scale: Development of a new measure. Poster presented at the annual meeting of the Society for Research on Nicotine and Tobacco, San Diego, CA.
Shiffman, S., Waters, A. J., & Hickcox, M. (2004). The Nicotine Dependence Syndrome Scale: A multidimensional measure of nicotine dependence. Nicotine & Tobacco Research, 6, 327-348. https://doi.org/10.1080/1462220042000202481 
Singh, A., Grau, E., & Folsom, R., Jr. (2002). Predictive mean neighborhood imputation for NHSDA substance use data. In J. Gfroerer, J. Eyerman, & J. Chromy (Eds.), Redesigning an ongoing national household survey: Methodological issues (HHS Publication No. SMA 03-3768, pp. 111-133). Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies.
Smith, R. V., Havens, J. R., & Walsh, S. L. (2016). Gabapentin misuse, abuse and diversion: A systematic review. Addiction, 111, 1160-1174. https://doi.org/10.1111/add.13324 
Solari, C. D., Cortes, A., Henry, M., Matthews, N., & Morris, S. (2014, October). The 2013 Annual Homeless Assessment Report (AHAR) to Congress. Part 2: Estimates of homelessness in the United States. Washington, DC: U.S. Department of Housing and Urban Development, Office of Community Planning and Development.
SRNT Subcommittee on Biochemical Verification. (2002). Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research, 4, 149-159. https://doi.org/10.1080/14622200210123581 
Substance Abuse and Mental Health Services Administration, Center for Mental Health Services. (1993, May 20). Final notice [Final definitions for: (1) Children with a serious emotional disturbance, and (2) adults with a serious mental illness]. Federal Register, 58(96), 29422-29425.
Substance Abuse and Mental Health Services Administration, Disaster Technical Assistance Center. (2021, May). A preliminary look at the mental health and substance use-related effects of the COVID-19 pandemic. Supplemental Research Bulletin.
Taylor, D. B. (2021, March 28). George Floyd protests: A timeline. The New York Times. Retrieved from https://www.nytimes.com/article/george-floyd-protests-timeline.html 
Torales, J., O’Higgins, M., Castaldelli-Maia, J. M., & Ventriglio, A. (2020). The outbreak of COVID-19 coronavirus and its impact on global mental health. International Journal of Social Psychiatry, 66(4), 317-320. https://doi.org/10.1177/0020764020915212 
Tourangeau, R., & Smith, T. W. (1996). Asking sensitive questions: The impact of data collection mode, question format, and question context. Public Opinion Quarterly, 60, 275-304. https://doi.org/10.1086/297751 
Tourangeau, R., & Yan, T. (2007). Sensitive questions in surveys. Psychological Bulletin, 133, 859-883. https://doi.org/10.1037/0033-2909.133.5.859 
Turner, C. F., Lessler, J. T., & Gfroerer, J. C. (Eds.). (1992). Survey measurement of drug use: Methodological studies (HHS Publication No. ADM 92-1929). Rockville, MD: National Institute on Drug Abuse.
Turner, C. F., Ku, L., Rogers, S. M., Lindberg, L. D., Pleck, J. H., & Sonenstein, F. L. (1998). Adolescent sexual behavior, drug use, and violence: Increased reporting with computer survey technology. Science, 280, 867-73. https://doi.org/10.1126/science.280.5365.867 
Underwood, J. M., Brener, N., Thornton, J., Harris, W. A., Bryan, L. N., Shanklin, S. L., Deputy, N., Roberts, A. M., Queen, B., Chyen, D., Whittle, L., Lim, C., Yamakawa, Y., Leon-Nguyen, M., Kilmer, G., Smith-Grant, J., Demissie, Z., Everett Jones, S., Clayton, H., & Dittus, P. (2020). Overview and methods for the Youth Risk Behavior Surveillance System — United States, 2019. Morbidity and Mortality Weekly Report, 69, 1-10. https://doi.org/10.15585/mmwr.su6901a1 
Ursano, R. J., Heeringa, S. G., Stein, M. B., Jain, S., Raman, R., Sun, X., Chiu, W. T., Colpe, L. J., Fullerton, C. S., Gilman, S. E., Hwang, I., Naifeh, J. A., Nock, M. K., Rosellini, A. J., Sampson, N. A., Schoenbaum, M., Zaslavsky, A. M., & Kessler, R. C. (2015). Prevalence and correlates of suicidal behavior among new soldiers in the U.S. Army: Results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). Depression and Anxiety, 32(1), 3-12. https://doi.org/10.1002/da.22317 
U.S. Census Bureau. (2020, September 15). 2019 National Survey of Children’s Health. Methodology report. Washington, DC: U.S. Department of Commerce, Economics and Statistics Administration, U.S. Census Bureau. Retrieved from https://census.gov/
U.S. Census Bureau. (2021, May 14). Experimental data products: What are experimental data products? Retrieved on June 14, 2021, from https://www.census.gov/data/experimental-data-products.html
U.S. Census Bureau. (n.d.). Household Pulse Survey. Expected loss in employment income, week 1, April 23-May 5, 2020. Retrieved on July 8, 2021, from https://www.census.gov/data-tools/demo/hhp/#/?measures=LFNEXT&periodSelector=1
U.S. Department of Health and Human Services. (2021a, May). What is telehealth? Health Resources & Services Administration. Retrieved from https://telehealth.hhs.gov/patients/understanding-telehealth/
U.S. Department of Health and Human Services. (2021b, July). Billing for telebehavioral health. Health Resources & Services Administration. Retrieved from https://telehealth.hhs.gov/providers/telehealth-for-behavioral-health/billing-for-telebehavioral-health/
U.S. Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response. (2020, January 31). Determination that a public health emergency exists. Retrieved from https://www.phe.gov/emergency/news/healthactions/phe/Pages/2019-nCoV.aspx
U.S. Drug Enforcement Administration. (2017). Drugs of abuse, a DEA resource guide. Retrieved from https://www.dea.gov/
U.S. Drug Enforcement Administration. (2020a). Controlled substances. Alphabetical order. Retrieved from https://www.deadiversion.usdoj.gov/
U.S. Drug Enforcement Administration. (2020b). Drugs of abuse, a DEA resource guide: 2020 edition. Retrieved from https://www.dea.gov/
U.S. Food and Drug Administration. (2017, May 16). Drug Safety and Risk Management Advisory Committee (DSaRM). Washington, DC: Author. Retrieved from https://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/default.htm
U.S. Food and Drug Administration. (2018, February 5). Understanding unapproved use of approved drugs “off label.” Washington, DC: Author. Retrieved from https://www.fda.gov/patients/learn-about-expanded-access-and-other-treatment-options/understanding-unapproved-use-approved-drugs-label
van den Berg, J. J., Adeyemo, S., Roberts, M. B., Bock, B. C., Stein, L. A. R., Martin, R. A., Parker, D. R., & Clarke, J. G. (2018). Comparing the validity of self-report and urinalysis for substance use among former inmates in the northeastern United States. Substance Use & Misuse, 53(10), 1756-1761. https://doi.org/10.1080/10826084.2018.1432646 
Vehovar, V., & Čehovin, G. (2014). Questionnaire length and breakoffs in web surveys: A meta study. Paper presented at the 7th Internet Survey Methodology Workshop. Bolzano, Italy. Retrieved from http://www.websm.org/db/12/17719/Web%20Survey%20Bibliography/Questionnaire_length_and_breakoffs_in_web_surveys_a_meta_study/ 
Wang, J., Sumner, S. A., Simon, T. R., Crosby, A. E., Annor, F. B., Gaylor, E., Xu, L., & Holland, K. M. (2020). Trends in the incidence and lethality of suicidal acts in the United States, 2006 to 2015. JAMA Psychiatry, 77, 684-693. https://doi.org/10.1001/jamapsychiatry.2020.0596 
Williams, C. L., Davidson, J. A., & Montgomery, I. (1980). Impulsive suicidal behavior. Journal of Clinical Psychology, 36(1), 90-94. https://doi.org/10.1002/1097-4679(198001)36:1<90::AID-JCLP2270360104>3.0.CO;2-F 
Wilson, N., Kariisa, M., Seth, P., Smith, H., & Davis, N. L. (2020). Drug and opioid-involved overdose deaths—United States, 2017-2018. Morbidity and Mortality Weekly Report, 69, 290-297. https://doi.org/10.15585/mmwr.mm6911a4 
Footnotes
1 RTI International is a trade name of Research Triangle Institute. RTI and the RTI logo are U.S. registered trademarks of Research Triangle Institute.
2 Small area estimation is a hierarchical Bayes modeling technique used to make state-level estimates for 32 measures related to substance use and mental health. For more details, see “2018-2019 National Survey on Drug Use and Health: Guide to State Tables and Summary of Small Area Estimation Methodology” at https://www.samhsa.gov/data/.
3 Table 2.1 also provides sample information to support a clinical validation study for 2020.
4 Sampling areas were defined using 2010 census geography. Counts of DUs and population totals were obtained from the 2010 decennial census data supplemented with revised population projections from Claritas.
5 Census tracts are relatively permanent statistical subdivisions of counties and parishes and provide a stable set of geographic units across decennial census periods.
6 Some census tracts had to be aggregated in order to meet the minimum DU requirement. In California, Florida, Georgia, Illinois, Michigan, New Jersey, New York, North Carolina, Ohio, Pennsylvania, Texas, and Virginia, this minimum size requirement was 250 DUs in urban areas and 200 DUs in rural areas. In the remaining states and the District of Columbia, the minimum requirement was 150 DUs in urban areas and 100 DUs in rural areas.
7 The composite measure of size is a weighted population size where the weights are the sampling rates defined for specified age groups.
8 The minimum DU size requirements for census tracts also were applied to census block groups. The purpose of the minimum DU size is to ensure that each sampled area has a sufficient number of DUs to field two NSDUH samples and one field test.
9 Eight segments per SSR per year were needed to field the 2014 through 2022 NSDUHs (8 segments × 9 years = 72 segments per SSR). For the 2015 through 2022 NSDUHs, half of the segments are carried over from the prior year (4 segments × 8 years = 32 segments per SSR). Thus, 40 unique segments per SSR were needed to field the 9-year sample (72 − 32 = 40).
10 Although data collection was suspended at the end of Quarter 1 and did not resume until Quarter 4, the hope was that field data collection could resume after a relatively short period of time. Consequently, the address samples still were selected on a quarterly basis.
11 The original target of 1,500 NSDUH respondents for the CVS sample was not achieved because of the suspension of NSDUH data collection in March 2020. There was no subsequent resumption of data collection for the CVS.
12 See Table 2.1 for the list of the 12 largest states and their targeted numbers of interviews (but not the final sample sizes by state).
13 The population sampling rates were lower than in previous years because the COVID-19 pandemic significantly affected data collection, response rates, and sample sizes in 2020. A power analysis was conducted, and the achieved sample for the 2020 NSDUH is sufficient for producing national estimates for people aged 12 or older and adults aged 18 or older.
14 Use of “illicit drugs” in NSDUH refers to the use of marijuana, cocaine, heroin, hallucinogens, inhalants, or methamphetamine, or the misuse of prescription pain relievers, tranquilizers, stimulants, or sedatives.
15 For brevity, the word “section” is used in the remainder of this report to refer to interview sections in the NSDUH questionnaire.
16 The original NSDUH sample target was initially planned to be supplemented with 1,500 NSDUH respondents across the first two calendar quarters of 2020 (i.e., January to June). Because of COVID-19, however, NSDUH data collection (including recruitment into the CVS) was suspended on March 16, 2020, and no data were collected during the second calendar quarter of 2020 (i.e., April to June). Therefore, the standard in-person data collection procedures described in Section 2.2.1.1.1 applied to the CVS data collection.
17 The new SUD module and the clinical follow-up interview were conducted only in English. Expert feedback indicated that it was unnecessary to conduct the validation study on both English and Spanish versions of the NSDUH SUD module. Therefore, respondents who chose to answer the NSDUH main study questions in Spanish were excluded from the CVS.
18 Respondents who reported no past year use of alcohol or illicit drugs were eligible for the CVS sample.
19 For CVS respondents, questions assessing DSM-IV symptoms also included in the DSM-5 criteria (e.g., legal troubles) were included at the end of the DSM-5 SUD module. For main study non-CVS respondents, questions assessing DSM-5 symptoms not also included in the DSM-IV criteria (e.g., craving and cannabis withdrawal) were included at the end of the CAI instrument.
20 For some dwelling units, the field lister could not determine a street address and provided a description of the dwelling unit. If selected for web-based data collection, dwelling units listed by description were not sent an introductory letter. Eligibility for these dwelling units was imputed for weighting and response rate calculations.
21 The web-based screening and interview instruments were available in English and Spanish but not other languages.
22 A gate question is an initial question or set of questions that ask whether the behavior or characteristic of interest applies to the respondent. An affirmative response to a gate question leads to respondents being asked a series of other related questions. A response other than an affirmative one to all relevant gate questions results in respondents skipping additional questions on that topic and being routed to the next set of topics in the interview.
23 In this text, “prescription drugs” refers to any prescription drug in a given category (e.g., any prescription pain reliever).
24 Past year or lifetime use of prescription drugs since 2015 refers to use for any reason (i.e., use of prescribed medication as directed or misuse of prescription drugs).
25 If all 2020 NSDUH had been collected from respondents in person (as in prior years), then assessing trends would have been relevant for 2020. Because of the methodological changes discussed in Sections 2.1 and 2.2, however, NSDUH tables and reports for 2020 do not present trend data.
26 Missing data for SUD variables in 2015 to 2019 were imputed using modified PMN for hallucinogens, inhalants, methamphetamine, and prescription drugs (i.e., prescription pain relievers, tranquilizers, stimulants, and sedatives). Beginning in 2020, missing data for SUD variables also were imputed using modified PMN for alcohol, marijuana, cocaine, and heroin.
27 In stochastic imputation, random numbers on the interval (0,1) are independently selected for each nonrespondent. Imputation values are then assigned based on the size of the random variable in respect to the respondent’s predicted mean. For instance, for a dichotomous variable, if the selected random number is less than the respondent’s predicted mean, a value of 1 is imputed. CSI reduces the probability of unusual results by spreading the random numbers evenly between 0 and 1. That is, the elements needing an imputed value are randomly sorted (with the order 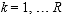 ); a random number,
); a random number,  , is independently chosen from the uniform distribution on the interval (0,1); and an imputed value of 1 is assigned for the element to be imputed with sorted index,
, is independently chosen from the uniform distribution on the interval (0,1); and an imputed value of 1 is assigned for the element to be imputed with sorted index, 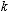 , if and only if the predicted mean is greater than
, if and only if the predicted mean is greater than 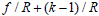 .
.
28 Variables for 2020 also were imputed using modified PMN for DSM-IV SUD outcomes for hallucinogens, inhalants, methamphetamine, and prescription drugs (i.e., prescription pain relievers, tranquilizers, stimulants, and sedatives). However, these DSM-IV SUD outcomes were not used in 2020 NSDUH tables or reports.
29 Person-level analysis weights refer to the weights used to produce population estimates from final survey respondents’ data. Other special weights also are produced for NSDUH (e.g., pair weights for analysis of data from pairs of responding household members), but this report does not discuss the creation of these special weights.
30 Poststratification of household weights to meet population controls for various household-level demographics was done to obtain census-consistent estimates based on the household rosters from all screened households.
31 This adjustment poststratified the weights of selected household members to conform to the adjusted roster estimates. This step took advantage of the separate screening and interviewing nature of the NSDUH design.
32 In some years, not all of the race domains in Table 3.1 are forced to fully match the U.S. Census Bureau population estimates due to some models not converging. When race domains do not fully match the U.S. Census Bureau population estimates, the sampling variation in 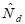 for these domains is considered negligible. Therefore, the race domains are considered fixed for every year.
for these domains is considered negligible. Therefore, the race domains are considered fixed for every year.
33 Because models were fit separately to create Quarter 1 and Quarter 4 analysis weights in 2020 (see Section 2.3.4.2), there were some changes to the main effects and the two-way interaction categories in 2020 (see Table 3.1). For the set of domains with a fixed 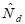 in 2019 (which were used for 2019 estimates in the 2020 detailed tables), see Table 3.1 of the 2019 National Survey on Drug Use and Health: Methodological Summary and Definitions (CBHSQ, 2020c).
in 2019 (which were used for 2019 estimates in the 2020 detailed tables), see Table 3.1 of the 2019 National Survey on Drug Use and Health: Methodological Summary and Definitions (CBHSQ, 2020c).
34 Starting in 2020 for confidentiality protection, survey sample sizes greater than 100 were rounded to the nearest 10, and sample sizes less than 100 were not reported (i.e., are shown as “<100” in tables).
35 The derivation for 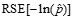 is
is 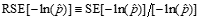 for
for 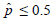 . The Taylor-series linearization of the numerator
. The Taylor-series linearization of the numerator 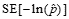 is
is 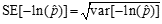 , which approximately equals
, which approximately equals 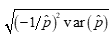 by Taylor-series linearization, which in turn equals
by Taylor-series linearization, which in turn equals 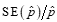 .
.
36 The suppression rule for prevalence rates, as shown in the first row of Table 3.2, presents the RSE rule expressed in terms of  and the effective n instead of
and the effective n instead of 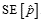 . The W-shaped plot in Figure 3.1 illustrates the RSE rule expressed in terms of
. The W-shaped plot in Figure 3.1 illustrates the RSE rule expressed in terms of 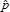 and the effective n. The effective n threshold was required to be a uniform 68 for
and the effective n. The effective n threshold was required to be a uniform 68 for 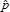 between 0.2 and 0.8, which is indicated by the horizontal line at effective n = 68. Based on the curve, the effective n threshold of n = 50 was determined to be too low for
between 0.2 and 0.8, which is indicated by the horizontal line at effective n = 68. Based on the curve, the effective n threshold of n = 50 was determined to be too low for 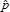 between 0.2 and 0.8, the points where the W shape double dips.
between 0.2 and 0.8, the points where the W shape double dips.
37 This statement applies to years prior to 2020 because no testing was done between estimates in different survey years for the 2020 NSDUH.
38 The degrees of freedom for most statistical tests are calculated as the number of primary sampling units (variance replicates) minus the number of strata. Because there are two replicates per stratum, 750 degrees of freedom equal the number of strata in the national sample for 2020. However, the degrees of freedom are smaller for some statistical comparisons; specifically, the degrees of freedom are reduced for estimates on the average number of days people used substances. Additionally, for the 2020 NSDUH, the degrees of freedom were reduced for estimates for individual quarters.
39 See footnote 37.
40 Other statistical methods have been used for comparisons of pairwise differences across three or more levels of a categorical variable once an overall test (such as Shah’s F) suggests there are differences. Although a Bonferroni adjustment can be applied to every pairwise difference (i.e., and not just to the pairwise difference with the lowest p value, which is sometimes recommended instead of Shah’s F as an alternative overall test), this is an overly conservative procedure. For example, if a p value of .05 is set as the criterion for statistical significance and there are three pairwise comparisons, then the Bonferroni-adjusted p value for statistical significance becomes .017 (i.e., .05 divided by 3 equals .017).
41 A successfully screened household is one in which all screening questionnaire items were answered by an adult resident of the household and either zero, one, or two household members were selected for the NSDUH interview.
42 Prior to 2002, the survey was known as the National Household Survey on Drug Abuse (NHSDA).
43 As an example of skip logic, adult respondents were asked in the adult mental health service utilization section if they received mental health treatment or counseling as an inpatient in the past 12 months. If respondents did not know or refused to report whether they received mental health services as an inpatient in that period, then questions were skipped for (1) the location where they received inpatient services, (2) the number of nights they spent in specific types of inpatient facilities, and (3) sources of payment for inpatient mental health care. Variables corresponding to these skipped questions had missing data.
44 Percentages of adult or adolescent respondents with missing data for lifetime symptoms of depression do not include weight gain because of pregnancy, which is asked only of females.
45 For brevity, percentages of respondents answering “don’t know” for perceived risk are presented for all respondents, regardless of the data collection mode. As indicated in the main text, responses of “don’t know” for the perceived risk of harm from substance use may suggest an underlying characteristic of respondents, independent of mode.
46 AMI and SMI are estimated based on a logistic regression model. Several measures in the model (e.g., psychological distress, serious thoughts of suicide, major depressive episode) treat missing data as being equivalent to negative responses (see Section 3.4.7).
47 Adults were asked to report the age when they first had a period of 2 weeks or longer when they were sad or discouraged or lost interest in most things for most of the day nearly every day and also reported they had some symptoms of depression. Adolescents were asked to report the age when they first had a period of 2 weeks or longer when they were sad, discouraged, or really bored and also reported they had some symptoms of depression.
48 The COVID-19 pandemic also could have posed health risks for others coming into contact with respondents or FIs, such as family members living with FIs.
49 As noted in Section 2.2.1.3, SDUs could start out in Quarter 4 as being selected for in-person data collection but transition to web-based data collection later in the quarter if county-level COVID-19 infection rates increased. However, SDUs in Quarter 4 could not start out as being selected for web-based data collection and transition to in-person data collection.
50 These special analysis weights did not need to be used for statistically imputed variables that had no missing data.
51 The set of 20 key substance use and mental health outcomes used in these analyses for respondents 12 or older include lifetime, past year, and past month marijuana use; lifetime, past year, and past month cigarette use; lifetime, past year, and past month alcohol use; lifetime, past year, and past month illicit drug use; and past month binge alcohol use. Outcomes for respondents 18 or older include: past year any mental illness, past year serious mental illness, past year major depressive episode, past year major depressive episode with severe impairment, serious thoughts of suicide in past year, made any suicide plans in past year, attempted suicide in past year.
52 Results of this study showed an 84.6 percent agreement between self-reported tobacco use in the past 30 days and urine drug test results for tobacco. For marijuana, there was 89.8 percent agreement between self-reported use in the past 30 days and urine drug test results, although this agreement was dominated by people who reported no use and tested negative (82.9 percent).
53 Exceptions are for the pain relievers OxyContin® (an extended-release formulation of oxycodone) and Zohydro® ER (an extended-release formulation of hydrocodone) because generic equivalents for these drugs were not available by prescription in the United States in 2020.
54 Prior to 2015, respondents were asked only about the misuse of specific prescription drugs. This previous question structure required respondents to think about multiple pieces of information in order to answer a single question.
55 The NSDUH questionnaire since 2015 has not asked about any use of prescription drugs in the past month.
56 As part of the editing procedures (Section 2.3.2), respondents who specified OTC drugs were the only other prescription drugs they misused in the past 12 months were logically inferred not to have misused other prescription drugs in that category (e.g., pain relievers) in the past year. Respondents also were logically inferred not to have misused any prescription drug in that category in the past year if they reported only the misuse of any other prescription drug in the past 12 months and reported OTC drugs were the only drugs they misused.
57 Since 2015, respondents have been asked about any use of prescription psychotherapeutic drugs. Any use includes use of medication as directed with a prescription of the individual’s own or misuse of prescription psychotherapeutics. Initiation for psychotherapeutics in NSDUH refers to the first time people misused these medications rather than the first time they used these medications for any reason.
58 For brevity, “misuse” is not repeated whenever the text refers to first use. Terms such as “past year use” and “first use” used in the remainder of this chapter for substance use in general refer to misuse for prescription psychotherapeutic drugs.
59 As noted in Section 3.3.3 and Chapter 6, SAMHSA decided not to compare estimates for 2020 with corresponding estimates from prior years, including those for the mean age at first use among past year initiates.
60 Respondents also were asked the follow-up question if the sum of the reports of past year initiation plus missing data for initiation equaled the number of specific drugs they misused in the past year (and there were no reports of initiation of misuse more than 12 months prior to the interview date).
61 The questionnaire included items for the age, year, and month of first misuse for each individual psychotherapeutic drug respondents misused in the past year. A day of first misuse was imputed for past year initiates.
62 The SUD variables based on the DSM-IV criteria will be available on the 2020 NSDUH public use data file. See the 2019 methodological summary and definitions report (CBHSQ, 2020c) for details on how SUD variables were created based on the DSM-IV criteria.
63 For alcohol, for example, withdrawal symptoms include (but are not limited to) trouble sleeping, hands trembling, hallucinations (seeing, feeling, or hearing things that were not really there), or feeling anxious.
64 Respondents in 2020 who did not receive the revised questions for the special Clinical Validation Study in Quarter 1 (see Section 3.4.3.4) were not asked about the use of a substance (or a related substance) to get over or avoid withdrawal symptoms.
65 This number does not include respondents whose status as past year alcohol users was unknown based on their questionnaire responses but who were statistically imputed to be past year alcohol users.
66 The workgroup was chaired by Terry Zobeck of ONDCP. Agencies participating included ONDCP, SAMHSA, NIDA, the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute of Justice, and the Bureau of Justice Statistics.
67 Respondents who were lifetime but not past year users of alcohol or illicit drugs could nevertheless report the receipt of assistance in the past year, such as attending self-help groups to maintain recovery from problems related to their prior substance use.
68 Illicit drugs included marijuana, cocaine, heroin, hallucinogens, inhalants, methamphetamine, and the misuse of prescription psychotherapeutic drugs (i.e., pain relievers, tranquilizers, stimulants, and sedatives).
69 As per the definition of the need for substance use treatment described previously, people who had an SUD were classified as needing substance use treatment.
70 These codes are also known as the Beale Codes.
71 These codes were first developed in 1974 and have been updated approximately every 10 years since then. They are available at https://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx by clicking on that page’s link to the “Rural-Urban Continuum Codes.”
72 See footnote 42.
73 The errors discussed in Section 3.3.5 were identified for 2007 and 2008 after the effects of changes to the questionnaire for 2008 had been investigated. As noted in Section 3.3.5, however, these errors had minimal impact on the national estimates. Therefore, the data errors affecting the data for 2007 and 2008 were unlikely to change the overall conclusions reached about the effects of these questionnaire changes on estimates for 2008.
74 Approximate adult sample size prior to the 2020 NSDUH.
75 Structured Clinical Interview for the DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP); clinical interviews would require the use of a DSM-5 diagnostic assessment to identify mental disorders according to DSM-5 criteria (First et al., 2002).
76 MDE also was included in the 2012 model and is discussed in more detail in Section 3.4.8.
77 The GAF is a numeric scale used by mental health clinicians to quantify the severity of mental disorders and the extent to which mental disorders negatively affected a person’s daily functioning. In the MHSS, GAF scores were assigned by clinical interviewers at the end of each SCID interview based on information gathered throughout the interview about symptoms of mental disorders and related impairment. This procedure differs from use of the WHODAS in NSDUH, which relies on respondents’ (rather than clinicians’) perceptions of the extent to which their symptoms of psychological distress affected their day-to-day functioning.
78 The number of final adult respondents differs from the number of interviews for adults presented in Tables 3.4 to 3.7 because the data in these tables are based on initial demographic information obtained from screener data.
79 Missing values in individual K6 items were assigned a value of zero for computing the imputation-revised K6 item scores.
80 In the question about serious thoughts of suicide (SUI01), “[DATEFILL]” refers to the date at the start of a respondent’s 12-month reference period. The interview program sets the start of the 12-month reference period as the same month and day as the interview date but in the previous calendar year.
81 Treating missing data for serious thoughts of suicide and past year MDE as being equivalent to negative responses applied only to the 2012 model. For published estimates for serious thoughts of suicide and past year MDE, respondents with missing data were excluded from the analyses. In addition, published 2020 estimates for serious thoughts of suicide and past year MDE among adults used the person-level break-off analysis weights described in Section 2.3.4.2.
82 Both the lifetime and past year measures of MDE in adults (see Section 3.4.8) were used in poststratification.
83 Past year MDE was estimated based on responses to the SCID from the MHSS respondents and on responses from all adults to the main survey (see Section 3.4.8). These two measures were created independently. The reference here is to the SCID measure from the MHSS.
84 In this situation, the past year MDE measure is from the main NSDUH interview (i.e., not from the SCID).
85 This was computed like SMI and AMI using the cut point probability 0.077686285365.
86 For details, see the following webpage: https://www.hcp.med.harvard.edu/ncs/  .
.
87 “An MDE” refers to the occurrence of at least one MDE, rather than only one MDE. Similarly, reference to “the MDE” in a given period (e.g., the past 12 months) does not mean an individual had only one MDE in that period.
88 This statement applies to years prior to 2020 because there was no testing between estimates in different survey years for the 2020 NSDUH.
89 Questions for the provision of virtual services for substance use treatment or medical services were included in sections of the 2020 NSDUH questionnaire in Quarter 4 that applied to all respondents aged 12 or older. Questions for the provision of virtual mental health services were asked separately for adults and adolescents.
90 Illicit drugs included marijuana, cocaine (including crack), heroin, hallucinogens, inhalants, methamphetamine, or prescription psychotherapeutics that were misused, which included prescription pain relievers, tranquilizers, stimulants, and sedatives.
91 Due to a partial redesign of the prescription drug section of the questionnaire in the 2015 NSDUH, a trend break occurred. Consequently, estimates for 2015 were no longer comparable with prior years’ estimates.
92 Estimates are presented for 2020 separately because methodological changes for the 2020 NSDUH (see Chapter 2) could affect whether the 2020 estimates are comparable with estimates from prior years.
93 Chapter 4’s figures and tables are presented together at the end of the chapter’s text discussion.
94 Product label information for Flexeril® is available on the FDA’s Center for Drug Evaluation and Research website at https://www.fda.gov/Drugs/. The product label for generic cyclobenzaprine is not available on the FDA website.
95 For example, the product label for Xanax®, which is prescribed as a tranquilizer, indicates the drug has an average half-life of 11.2 hours (i.e., the length of time for half of the dosage of the drug to be metabolized), with a range of 6.3 to 26.9 hours in healthy adults. In comparison, the product label for Halcion®, which is a benzodiazepine prescribed as a sedative, has a short half-life in the range of 1.5 to 5.5 hours. Product label information for these drugs is available on the FDA’s Center for Drug Evaluation and Research website at https://www.fda.gov/Drugs/.
96 When a drug is metabolized, it is converted into metabolites, which are the substances that remain after the drug is broken down by the body. For more information, see the definition for “metabolite” by typing this word as a search term on the MedlinePlus web page at https://www.nlm.nih.gov/medlineplus/.
97 Because of the general principle of not using data from one section of the interview to edit variables in another section (see Section 2.3.2.1), reports of Desoxyn® outside of the stimulants section are not used to infer the use and misuse of amphetamines.
98 The estimated total will be lower than the true population total if the negative bias from excluding respondents with missing data outweighed other potential sources of random error (e.g., sampling error resulting from the selection of a sample) or nonrandom error (e.g., overreporting of the characteristic) that affected estimated totals in a positive direction.
99 An exception to this general principle applied to respondents who specified they misused one or more prescription drugs for a given subtype as some “other” prescription drug they misused in the past year. For example, suppose respondents answered “don’t know” when presented with the list of hydrocodone products for any use in the past year. If these respondents reported the misuse of “other” pain relievers in the past year and then specified a hydrocodone product (e.g., Vicodin®) was one of the other prescription pain relievers they misused in the past year, then these respondents logically misused hydrocodone products in the past year. These respondents also logically used hydrocodone products in the past year for any reason.
100 “Off label” prescribing refers to the prescribing of a drug that has been approved for use in the United States, but the drug is being prescribed for a condition the drug is not approved to treat (FDA, 2018).
101 The following states are in HHS Region 4: Alabama, Florida, Georgia, Kentucky, Mississippi, North Carolina, South Carolina, and Tennessee.
102 Nonopioid drugs included prescription pain relievers that are not opioids, prescription drugs other than pain relievers, illicit drugs other than heroin or other opioids, and OTC drugs. Specified responses for other pain relievers that were given a nonspecific code (i.e., “analgesic, not specified,” “Don’t know,” or “refused”) were treated as potential indications of opioid misuse for this analysis.
103 For simplicity, respondents who were statistically imputed to have misused prescription pain relievers in the past year without providing information about specific pain relievers they misused also were assumed to have misused prescription opioids.
104 Although prescription opioids also cause drowsiness, they do not act on the brain in the same way as tranquilizers or sedatives (NIDA, 2020a).
105 Although measures of the use of tranquilizers or sedatives in the past 12 months for any reason (i.e., use of prescriptions of one’s own as directed by a doctor or misuse of these medications) can be created from the NSDUH data since 2015, these measures were not created for NSDUH reports and tables since 2018.
106 See the 2020 NSDUH questionnaire specifications available at https://www.samhsa.gov/data/.
107 A similar question structure was used for respondents who did not report any past year use of tranquilizers.
108 The BRFSS website may not count states as administering an optional module if they administered it to less than the full sample of respondents in that state.
109 Since 2015, NSDUH and BRFSS have used nearly the same definition of binge alcohol use (i.e., four or more drinks on an occasion for females and five or more drinks on an occasion for males on at least 1 day [for NSDUH] or at least once [for BRFSS] in the past 30 days).
110 The BRFSS Web Enabled Analysis Tool (WEAT) is available by clicking on the “Prevalence Data & Data Analysis Tools” link at https://www.cdc.gov/brfss/.
111 In preparation for the transition to the administration of the questionnaire on electronic tablets in 2020, schools in 2019 were randomly assigned for administration of the survey using the traditional paper questionnaire or electronic tablets (Miech et al., 2021).
112 Prior to 2002, respondents were surveyed every other year until the age of 31 or 32 (i.e., up to seven times after graduation). In 2002, the seventh biennial follow-up was discontinued, with respondents being surveyed every other year until they reach the age of 29 or 30. Additional follow-ups then occur at 5-year intervals at ages 35, 40, 45, 50, 55, and 60; follow-up of 60-year-olds began in 2018.
113 Responses from both questionnaire modes were pooled together for the MTF substance use estimates in 2019 because the researchers found negligible differences in substance use estimates between the two modes (Johnston et al., 2020).
114 Testing for statistically significant differences between NSDUH and MTF estimates for adolescents was not conducted for this report because the NSDUH estimates are weighted estimates for adolescents aged 12 to 17, whereas MTF estimates are simple averages for 8th and 10th graders. However, in a report where formal statistical testing was done for substance use estimates among adolescents in NSDUH and MTF, the NSDUH estimates for the use of illicit drugs generally were lower than the MTF estimates (CBHSQ, 2012b).
115 Data on the percentages of adolescents and young adults who were not currently enrolled in school and had not graduated from high school are available at https://www.census.gov/. The CPS questionnaire (also available at https://www.census.gov/) indicates that high school graduates received a high school diploma or the equivalent.
116 Federal agencies participating in the Household Pulse Survey included the Bureau of Labor Statistics, Bureau of Transportation Statistics, Centers for Disease Control and Prevention, Department of Defense, Department of Housing and Urban Development, Maternal and Child Health Bureau, National Center for Education Statistics, National Center for Health Statistics, National Institute for Occupational Safety and Health, Social Security Administration, and U.S. Department of Agriculture Economic Research Service.
117 The following were the adapted PHQ-2 questions: “Over the last 7 days, how often have you been bothered by … having little interest or pleasure in doing things? Would you say not at all, several days, more than half the days, or nearly every day?” and “Over the last 7 days, how often have you been bothered by … feeling down, depressed, or hopeless? Would you say not at all, several days, more than half the days, or nearly every day?”
The following were the adapted GAD-2 questions: “Over the last 7 days, how often have you been bothered by the following problems … Feeling nervous, anxious, or on edge? Would you say not at all, several days, more than half the days, or nearly every day?” and “Over the last 7 days, how often have you been bothered by the following problems … Not being able to stop or control worrying? Would you say not at all, several days, more than half the days, or nearly every day?”
118 V codes denote conditions that are a focus of clinical attention or treatment but are not attributable to a mental disorder (e.g., marital problems).
119 For example, questionnaire data on the use of prescription medications in the past 30 days became available on the 2015-2016 NHANES public use data file in January 2019.
120 Data collected from 2019 to March 2020 were combined with data from the NHANES 2017-2018 cycle to form a nationally representative sample of NHANES 2017-March 2020 pre-COVID-19 pandemic data. See “NHANES 2017-March 2020” at https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/.
121 An eligible respondent for the family questionnaire is a family member (i.e., household member related by blood, marriage, or adoption to the head of the family) who is at least 18 years old. In families where there is no one aged 18 or older, interviewers are instructed to choose the head of the family or any person in the family who has ever been married as the respondent for the family questionnaire.
122 Asian translations for the introduction to the ACASI component appear in the Asian Interpreter notebook; the interpreter uses a hard-copy form to read the appropriate text to the respondent. On-screen translations are available in these Asian languages for the ACASI drug use section.
123 As noted previously, a proxy respondent provided information for NHANES respondents aged 12 to 15 or for those aged 16 or older who could not answer for themselves. For brevity, this discussion assumes that the questions apply to an NHANES respondent’s own prescription drug use.
124 Respondents are asked to show the NHANES interviewer all of the containers for the prescription medications that they took, and interviewers enter the names of the medications from the labels into the computer. If no container is available, the respondent reports the name of the drug to the interviewer.
125 The number of respondents aged 12 or older with prescription drug information in the 2013 and 2014 NHANES is lower than the number of respondents who completed the household interview because data on prescription drug use are collected for sample members younger than the age of 12.
126 In most states, the age of legal majority is 18, but in Alabama, Mississippi, and Nebraska, this age is older. However, all household members aged 18 or older who are at home at the time of the interview may respond for themselves for the NHIS Family Core component.
127 Spanish-speaking household members who request to be interviewed over the telephone are assigned to a trained agent who answers any questions and enters data into the Spanish-language web instruments.
128 National-level NSCH data can be analyzed online at https://www.childhealthdata.org/  by selecting “National Survey of Children’s Health Interactive Data Query,” then choosing a survey year and “Nationwide” in the field for the state/region. Data on current depression for a given year of the NSCH are available by selecting “Physical, Oral Health and Functional Status” from “Child and Family Health Measures,” then selecting “Prevalence of current depression, age 3-17 years” from the list of topics for “Prevalence of current or lifelong health conditions — list of 27 conditions.” The online analysis tool allows estimates to be shown by age group. The NSCH recommends using the 2-year combined data (e.g., 2018-2019) for the most reliable estimates.
by selecting “National Survey of Children’s Health Interactive Data Query,” then choosing a survey year and “Nationwide” in the field for the state/region. Data on current depression for a given year of the NSCH are available by selecting “Physical, Oral Health and Functional Status” from “Child and Family Health Measures,” then selecting “Prevalence of current depression, age 3-17 years” from the list of topics for “Prevalence of current or lifelong health conditions — list of 27 conditions.” The online analysis tool allows estimates to be shown by age group. The NSCH recommends using the 2-year combined data (e.g., 2018-2019) for the most reliable estimates.
129 All 2019 NSDUH data were collected in person.
130 NSDUH’s measurements include specific symptoms of depression, frequency of symptoms, and interference of depression with adolescents’ life activities (see Section 3.4.8 in this report). The NSCH measured whether the parent was told that the child had depression and the parent’s self-assessment of the severity of current depression.
131 The weighted 2019 NSDUH response rate is given for adults because parents completed the NSCH.
132 Counts of the number of people in treatment on a single day in N-SSATS were based on reports of the number of people in treatment on the last working day of March. Corresponding NSDUH estimates were based on data from respondents from the 2008 to 2010 NSDUHs who reported that they were enrolled in a specialty substance use treatment program on October 1 of the year prior to the interview or those from the 2007 to 2009 NSDUHs who were in specialty substance use treatment at the time of the interview (Batts et al., 2014).
133 The numbers of people in TEDS who received treatment were derived from linked admissions and discharge data or from adjusted admissions data for states that did not submit discharge data. Multiple admissions that were linked by a single unique identifier represented one individual. Three states (Alabama, Alaska, and Georgia) and the District of Columbia were not included in the TEDS data because they did not report TEDS data or reported incomplete data. For comparison purposes, data from these states were excluded from NSDUH data on average numbers who received treatment in the past year. However, single-day counts for people in treatment from N-SSATS and NSDUH included data from these states (Batts et al., 2014).
134 NCS-R respondents also were excluded from the analysis if they self-reported being ineligible for Army service because of histories of criminal behaviors, severe physical disorders or handicaps, or severe mental illness.
135 An active resident is a resident whose most recent assessment transaction was not a discharge and whose most recent transaction had a target date (assessment reference date for an assessment record or entry date for an entry record) fewer than 150 days old. If a resident did not have a transaction for 150 days, then that resident was assumed to have been discharged.
136 This selection was based on adult confinement facilities identified in the 2005 Census of State and Federal Adult Correctional Facilities, supplemented with updated information from websites maintained by each state’s department of corrections.
137 Federal facilities were grouped together and treated like a state for sampling purposes.
138 A shorter paper-and-pencil interviewing (PAPI) questionnaire was available for inmates who were unable to come to the private interviewing room or interact with the computer. In the NIS-3, 1.9 percent of prisoner interviews and 0.5 percent of jail inmate interviews were completed using the PAPI questionnaire (Bronson & Berzofsky, 2017).
139 For comparisons with the prison population, NSDUH estimates for adults were standardized to the prison population based on gender, race, Hispanic origin, and age. For comparisons with the jail population, NSDUH estimates were standardized to the jail population based on these same demographic characteristics.
140 Estimates in Household Pulse Survey data tables were not aggregated by phase or across phases.
141 To reinforce this point, the U.S. Census Bureau provides information and results from the Household Pulse Survey on its webpage for “Experimental Data Products,” which is available at https://www.census.gov/data/experimental-data-products/household-pulse-survey.html.
142 Sections for the use of mental health services and mental issues among adolescents followed the youth experiences section.
143 Interviewer-administered questions from in-person data collection needed to be modified for web-based data collection (Section 2.2.2.4), but the basic content of the questions did not change.
144 The percentage cited in Section 6.2.2.3 was for all respondents in Quarter 4.
145 Because 2020 Quarter 4 estimates used preliminary weights, estimates for prior years also used corresponding preliminary weights and imputation procedures for comparability.
146 The variables (and reference values) were mode of completion (in person), year of data collection (2019), COVID-19 positivity rate in the county (0 for 2019), the categorical household size (one or two people as the reference group; remaining categories for additional number of people in household), age group, gender, race, Hispanic origin, education for adults aged 18 or older, employment status for adults aged 18 or older, region, and county type.
147 California issued the first statewide stay-at-home order on March 19, 2020 (Moreland et al., 2020). NSDUH suspended in-person data collection on March 16, 2020.
Appendix A Footnotes
1 The 2020 NSDUH questionnaire is available at https://www.samhsa.gov/data/.
2 See the following reference: Center for Behavioral Health Statistics and Quality. (2020). 2019 National Survey on Drug Use and Health: Methodological summary and definitions. Retrieved from https://www.samhsa.gov/data/
3 See footnote 1.
4 See the following reference: American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5) (5th ed.). Washington, DC: Author.
5 See the following reference: American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (DSM-IV) (4th ed.). Washington, DC: Author.
6 See the following reference: Office of Management and Budget. (1997). Revisions to the standards for the classification of federal data on race and ethnicity. Federal Register, 62(210), 58781-58790.
7 See footnote 1.
8 See the reference in footnote 4.
9 See the reference in footnote 4.
10 See the reference in footnote 4.
11 See the reference in footnote 4.
12 See the reference in footnote 4.
13 See the reference in footnote 5.
14 These codes are updated approximately every 10 years and are available at https://www.ers.usda.gov/topics/rural-economy-population/rural-classifications.aspx by clicking on that page’s link to the “Rural/Urban Continuum Codes.”
15 Definitions of MSAs and micropolitan statistical areas as defined by the OMB are available by conducting a search at the following webpage: https://www.census.gov/.
16 See “Why is the disease being called coronavirus 2019, COVID-19?” at https://www.cdc.gov/coronavirus/2019-ncov/faq.html.
17 Respondents in 2002 to 2015 were asked specifically about driving under the influence of “illegal” drugs. However, respondents’ perceptions of what constitutes an “illegal” drug may differ depending on the marijuana laws in the states where respondents are living. Therefore, these questions were revised starting with the 2016 NSDUH as indicated in the definition above.
18 See the following reference: Endicott, J., Spitzer, R. L., Fleiss, J. L., & Cohen, J. (1976). The Global Assessment Scale: A procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry, 33, 766-771. https://doi.org/10.1001/archpsyc.1976.01770060086012 
19 See footnote 1 for the location of the 2020 NSDUH questionnaires.
20 For more information, see the following webpage: https://www.census.gov/.
21 See the reference in footnote 5.
22 In the recency-of-use question, “any hallucinogen” is the default wording except in special situations. For more information, see the link for the 2020 NSDUH questionnaire in footnote 1.
23 See the reference in footnote 4.
24 See the reference in footnote 5.
25 See the reference in footnote 4.
26 See the reference in footnote 5.
27 See the reference in footnote 6.
28 See the reference in footnote 4.
29 See the following reference: U.S. Drug Enforcement Administration. (2017). Drugs of abuse, a DEA resource guide. Retrieved from https://www.dea.gov/
30 See the reference in footnote 29.
31 See the reference in footnote 4.
32 See the reference in footnote 5.
33 For prescription psychotherapeutic drugs, substance use initiation refers to misusing any drug in that category for the first time in the past 12 months. Starting in 2015, respondents were asked about any use of prescription drugs in the past 12 months or in their lifetime (i.e., not necessarily misuse). However, respondents who reported any use of prescription drugs were not asked when they first used these drugs.
34 See the following reference: Kessler, R. C., Barker, P. R., Colpe, L. J., Epstein, J. F., Gfroerer, J. C., Hiripi, E., Howes, M. J., Normand, S. L., Manderscheid, R. W., Walters, E. E., & Zaslavsky, A. M. (2003). Screening for serious mental illness in the general population. Archives of General Psychiatry, 60, 184-189. https://doi.org/10.1001/archpsyc.60.2.184 
35 See the following reference: Leon, A. C., Olfson, M., Portera, L., Farber, L., & Sheehan, D. V. (1997). Assessing psychiatric impairment in primary care with the Sheehan Disability Scale. International Journal of Psychiatry in Medicine, 27(2), 93-105. https://doi.org/10.2190/t8em-c8yh-373n-1uwd 
36 See the following reference: Novak, S. P., Colpe, L. J., Barker, P. R., & Gfroerer, J. C. (2010). Development of a brief mental health impairment scale using a nationally representative sample in the USA. International Journal of Methods in Psychiatric Research, 19(Suppl. 1), 49-60.
37 See the following reference: Rehm, J., Üstün, T. B., Saxena, S., Nelson, C. B., Chatterji, S., Ivis, F., & Adlaf, E. (1999). On the development and psychometric testing of the WHO screening instrument to assess disablement in the general population. International Journal of Methods in Psychiatric Research, 8, 110-123. https://doi.org/10.1002/mpr.61 
38 See the reference in footnote 29.
39 See the reference in footnote 4.
40 See the following reference: Center for Behavioral Health Statistics and Quality. (2012). Results from the 2011 National Survey on Drug Use and Health: Summary of national findings (HHS Publication No. SMA 12-4713, NSDUH Series H-44). Rockville, MD: Substance Abuse and Mental Health Services Administration.
41 See the following reference: Center for Behavioral Health Statistics and Quality. (2020). National Survey on Drug Use and Health: 2019 public use file and codebook. Retrieved from https://datafiles.samhsa.gov/
42 See the reference in footnote 35.
43 See the reference in footnote 4.
44 See the reference in footnote 5.
45 See the reference in footnote 5.
46 See the following reference: First, M. B., Spitzer, R. L., Gibbon, M., & Williams, J. B. W. (2002). Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP). New York, NY: New York State Psychiatric Institute, Biometrics Research.
47 See the following reference: Alcohol, Drug Abuse, and Mental Health Administration (ADAMHA) Reorganization Act, Pub. L. No. 102-321 (1992).
48 See the reference in footnote 5.
49 See the reference in footnote 4.
50 See the reference in footnote 5.
51 See the reference in footnote 6.
52 See the following reference: Shiffman, S., Hickcox, M., Gnys, M., Paty, J. A., & Kassel, J. D. (1995, March). The Nicotine Dependence Syndrome Scale: Development of a new measure. Poster presented at the annual meeting of the Society for Research on Nicotine and Tobacco, San Diego, CA.
53 See the following reference: Shiffman, S., Waters, A. J., & Hickcox, M. (2004). The Nicotine Dependence Syndrome Scale: A multidimensional measure of nicotine dependence. Nicotine & Tobacco Research, 6, 327-348. https://doi.org/10.1080/1462220042000202481 
54 See the following reference: Fagerstrom, K.-O. (1978). Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addictive Behaviors, 3-4, 235-241. https://doi.org/10.1016/0306-4603(78)90024-2 
55 See the following reference: Heatherton, T. F., Kozlowski, L. T., Frecker, R. C., & Fagerstrom, K.-O. (1991). The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction, 86, 1119-1127. https://doi.org/10.1111/j.1360-0443.1991.tb01879.x 
56 See the reference in footnote 4.
57 See the reference in footnote 4.
58 See the reference in footnote 5.
59 See the following reference: Center for Behavioral Health Statistics and Quality. (2016). 2015 National Survey on Drug Use and Health: Methodological summary and definitions. Retrieved from https://www.samhsa.gov/data/
60 See the reference in footnote 59.
61 See the reference in footnote 4.
62 See the reference in footnote 6.
63 Respondents were not asked about treatment for prescription pain relievers, prescription tranquilizers, prescription stimulants, or prescription sedatives if they had not misused these substances in their lifetime.
64 See the reference in footnote 4.
65 See the reference in footnote 5.
66 See the reference in footnote 59.
67 For a description and properties of the K6 scale, see the reference in footnote 34.
68 More information about the creation of the statistically adjusted SPD variables can be found in the following two references:
Center for Behavioral Health Statistics and Quality. (2012). 2010 National Survey on Drug Use and Health: Methodological resource book (Section 16b, Analysis of effects of 2008 NSDUH questionnaire changes: Methods to adjust adult MDE and SPD estimates and to estimate SMI in the 2005-2009 surveys). Rockville, MD: Substance Abuse and Mental Health Services Administration.
Office of Applied Studies. (2009b). Results from the 2008 National Survey on Drug Use and Health: National findings (HHS Publication No. SMA 09-4434, NSDUH Series H-36). Rockville, MD: Substance Abuse and Mental Health Services Administration.
69 See the reference in footnote 41.
70 See the reference in footnote 35.
71 See the reference in footnote 59.
72 See the reference in footnote 4.
73 See the reference in footnote 5.
74 See the reference in footnote 4.
75 See the reference in footnote 59.
76 Data for cigarettes, smokeless tobacco, and cigars were available for the lifetime, past year, and past month periods. Data for pipe tobacco were available only for the lifetime and past month periods.
77 See the reference in footnote 4.
78 See the reference in footnote 4.
79 See the reference in footnote 5.
80 Respondents were asked about treatment for depression regardless of whether they were classified as having a major depressive episode (MDE). To produce estimates of treatment for depression among people with MDE, the analysis needs to be restricted to respondents who had a lifetime or past year MDE.
81 See the references in footnotes 36 and 37.
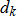 , to reduce bias due to nonresponse, to poststratify to known population control totals, and to control for extreme weights when necessary.
, to reduce bias due to nonresponse, to poststratify to known population control totals, and to control for extreme weights when necessary.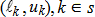 , for the adjustment factor
, for the adjustment factor 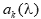 as follows:
as follows: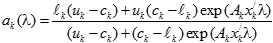
 are prespecified centering constants, such that
are prespecified centering constants, such that  and
and  . The variables
. The variables 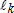 ,
, 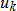 are unit-specific bounds, and
are unit-specific bounds, and 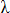 is the column vector of model parameters corresponding to the covariates in vector
is the column vector of model parameters corresponding to the covariates in vector 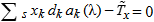
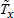 denotes control totals that could be either nonrandom, as is generally the case with poststratification, or random, as is generally the case for nonresponse adjustment.
denotes control totals that could be either nonrandom, as is generally the case with poststratification, or random, as is generally the case for nonresponse adjustment.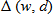 , defined as
, defined as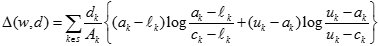
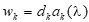 . This general approach was used at several stages of the weight adjustment process, including (1) adjustment of household weights for nonresponse at the screener level, (2) poststratification of household weights to meet population controls for various household-level demographics by state,
. This general approach was used at several stages of the weight adjustment process, including (1) adjustment of household weights for nonresponse at the screener level, (2) poststratification of household weights to meet population controls for various household-level demographics by state,
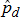 , such as drug use prevalence estimates for a domain
, such as drug use prevalence estimates for a domain 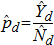
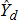 is a linear statistic estimating the number of people with the characteristic of interest (e.g., substance users) in the domain
is a linear statistic estimating the number of people with the characteristic of interest (e.g., substance users) in the domain 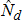 is a linear statistic estimating the total number of people in domain
is a linear statistic estimating the total number of people in domain 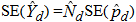
 ), or rates, within the range [
), or rates, within the range [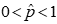 ], and the corresponding estimated numbers of users were suppressed if
], and the corresponding estimated numbers of users were suppressed if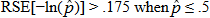
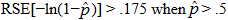
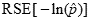 and
and 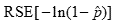 , the following equation was derived and used for computational purposes when applying a suppression rule dependent on effective sample size:
, the following equation was derived and used for computational purposes when applying a suppression rule dependent on effective sample size: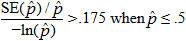
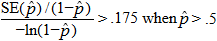
 and
and  produce a symmetric suppression rule; that is, if
produce a symmetric suppression rule; that is, if  will be suppressed as well (see
will be suppressed as well (see  , the symmetric properties of the rule produce a local minimum effective sample size of 50 at
, the symmetric properties of the rule produce a local minimum effective sample size of 50 at 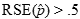 . This rule was changed because the previous RSE rule imposed a very stringent application for suppressing estimates when
. This rule was changed because the previous RSE rule imposed a very stringent application for suppressing estimates when 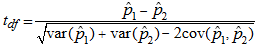
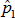 = the
= the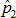 = the
= the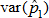 = the
= the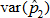 = the
= the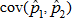 = the
= the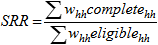
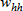 is the inverse of the unconditional probability of selection for the household and excludes all adjustments for nonresponse and poststratification defined in
is the inverse of the unconditional probability of selection for the household and excludes all adjustments for nonresponse and poststratification defined in 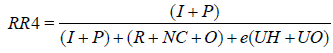
 , or
, or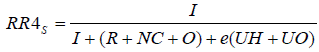
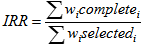
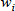 is the inverse of the probability of selection for the person and includes household-level nonresponse and poststratification adjustments (adjustments 1, 2, and 3 in
is the inverse of the probability of selection for the person and includes household-level nonresponse and poststratification adjustments (adjustments 1, 2, and 3 in 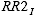 , which is based on the AAPOR definition, or
, which is based on the AAPOR definition, or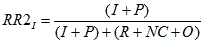
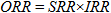 ,
, 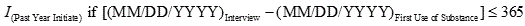
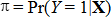 , the 2012 SMI prediction model was as follows:
, the 2012 SMI prediction model was as follows: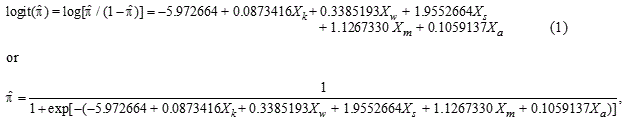
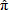 refers to the estimate of the SMI response probability
refers to the estimate of the SMI response probability  .
.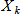 =
= 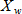 =
= 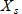 =
= 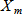 =
= 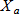 =
= 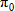 was determined, so that if
was determined, so that if 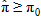 for a particular respondent, then the respondent was predicted to be SMI positive; otherwise, the respondent was predicted to be SMI negative. The cut point (0.260573529) was chosen so that the weighted numbers of false positives and false negatives in the MHSS dataset were as close to equal as possible. The predicted SMI status for all adult NSDUH respondents was used to compute prevalence estimates of SMI.
for a particular respondent, then the respondent was predicted to be SMI positive; otherwise, the respondent was predicted to be SMI negative. The cut point (0.260573529) was chosen so that the weighted numbers of false positives and false negatives in the MHSS dataset were as close to equal as possible. The predicted SMI status for all adult NSDUH respondents was used to compute prevalence estimates of SMI.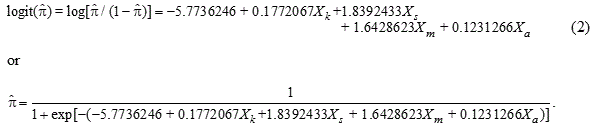
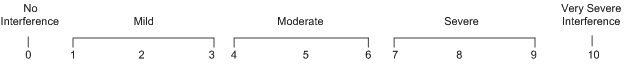
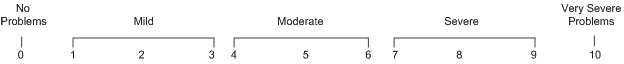
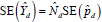 , is applied when the domain size estimates,
, is applied when the domain size estimates, 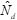 , are among those forced to match their respective U.S. Census Bureau or American Community Survey (ACS) population estimates through the weight calibration process.
, are among those forced to match their respective U.S. Census Bureau or American Community Survey (ACS) population estimates through the weight calibration process.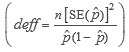
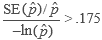 when
when 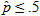 , or
, or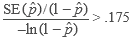 when
when 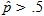 , or
, or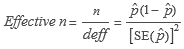 , or
, or , with nominal sample size,
, with nominal sample size, 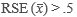 , or
, or
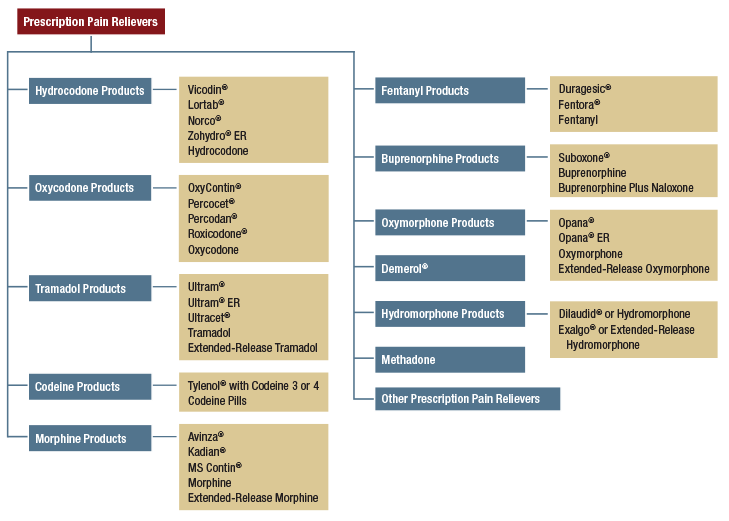
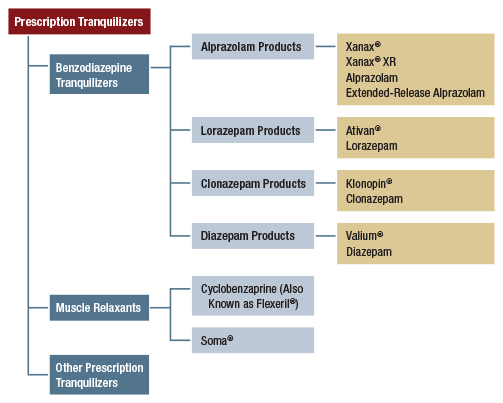
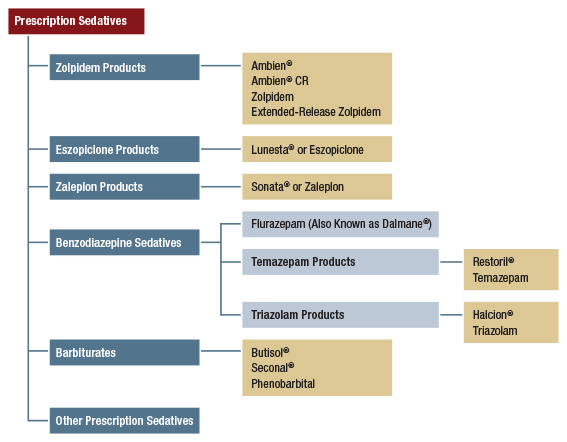





 ); a random number,
); a random number,  , is independently chosen from the uniform distribution on the interval (0,1); and an imputed value of 1 is assigned for the element to be imputed with sorted index,
, is independently chosen from the uniform distribution on the interval (0,1); and an imputed value of 1 is assigned for the element to be imputed with sorted index,  , if and only if the predicted mean is greater than
, if and only if the predicted mean is greater than 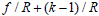 .
.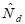 for these domains is considered negligible. Therefore, the race domains are considered fixed for every year.
for these domains is considered negligible. Therefore, the race domains are considered fixed for every year.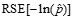 is
is 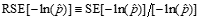 for
for 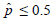 . The Taylor-series linearization of the numerator
. The Taylor-series linearization of the numerator 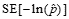 is
is 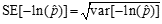 , which approximately equals
, which approximately equals 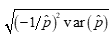 by Taylor-series linearization, which in turn equals
by Taylor-series linearization, which in turn equals 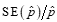 .
.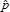 and the effective
and the effective 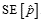 . The W-shaped plot in
. The W-shaped plot in 